Barbituric derivatives are a green option for photothermal applications.
In brief:
- The BoostCrop consortium has developed a series of barbituric acid derivatives with optimal properties for light-to-heat conversion.
- They are photostable, non-toxic, and can be synthesized sustainably.
The consortium BoostCrop, led by Vas Stavros in Warwick, has been set to develop molecular light-to-heat converters for agriculture. Their function is to protect crops from extreme cold weather and extend both the growing season and the geographic areas capable of supporting growth, all of which could help reduce food security challenges.
After two years of intense work, the BoostCrop teams have developed a new series of phenolic-based barbituric, which can successfully perform UV-to-heat conversion, satisfying all strict criteria set for agricultural applications.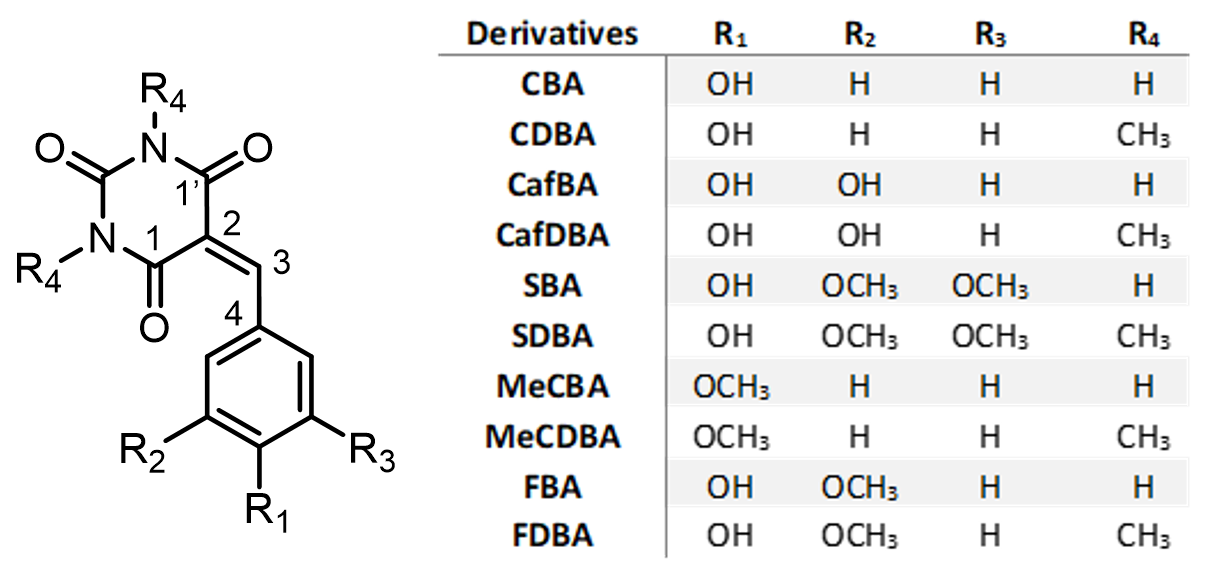 It has been a tour de force involving several research teams in the UK, Netherlands, and France, with experts on synthesis, steady-state and time-resolved spectroscopy, computational chemistry, and toxicology. Our team in Marseille, led by Josene Toldo, has been responsible for the computational chemistry part.
It has been a tour de force involving several research teams in the UK, Netherlands, and France, with experts on synthesis, steady-state and time-resolved spectroscopy, computational chemistry, and toxicology. Our team in Marseille, led by Josene Toldo, has been responsible for the computational chemistry part.
Following photoexcitation to the lowest singlet excited state, the barbituric absorbers repopulate the electronic ground state with high fidelity on an ultrafast time scale (within a few picoseconds). The energy relaxation pathway includes a twisted intramolecular charge-transfer state as the system evolves out of the Franck-Condon region, internal conversion to the ground electronic state, and subsequent vibrational cooling.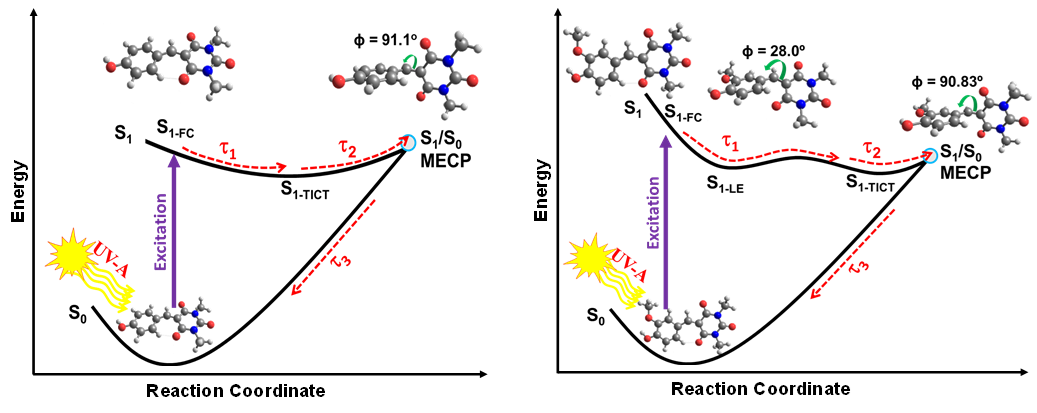 This fast, integral internal conversion is an essential feature we are looking for. Moreover, an important attribute of these barbiturics is the redshifted UV-absorption into the UV-A, in stark contrast to the many similar organic molecules studied previously.
This fast, integral internal conversion is an essential feature we are looking for. Moreover, an important attribute of these barbiturics is the redshifted UV-absorption into the UV-A, in stark contrast to the many similar organic molecules studied previously.
The barbiturics have been synthesised through catalyst- and organic solvent-free Knoevenagel condensation. This approach offers a sustainable and environmentally friendly synthetic route of barbiturics, which could be extended to other similar
systems.
Furthermore, in silico data have shown that these barbiturics do not show any critical potential for toxicity or genotoxicity.
Considering their green synthesis, impressive photostability, and lack of critical toxicity, the phenolic barbituric acid derivatives introduced in this work are promising molecular heaters for applications in agriculture (particularly when incorporated in a foliar spray to promote crop heating), phototherapy, photoimaging, and generally where photothermal conversion is desirable.
We have published these results in a paper in Chemical Science. In the words of one of the reviewers:
The study is a fine representation of what I have been expecting from the photodynamics community for many years – i.e. to use the knowledge gained from how small prototypical molecules behave upon photoexcitation and use it to synthetize a molecular system with the ultimate photochemical fidelity. It is a fantastic example of how this community has advanced across the past two decades. I am particularly impressed by the connection between the chemical physics, chemical synthesis, toxicology, and computational chemistry communities.
MB
Reference
[1] T. T. Abiola, B. Rioux, J. M. Toldo, J. Alarcan, J. M. Woolley, M. A. P. Turner, D. J. L. Coxon, M. T. d. Casal, C. Peyrot, M. M. Mention, W. J. Buma, M. N. R. Ashfold, A. Braeuning, M. Barbatti, V. G. Stavros, and F. Allais, Towards Developing Novel and Sustainable Molecular Light-to-Heat Converters, Chem. Sci. (2021), DOI: 10.1039/D1SC05077J