Computational chemistry explains a photochemical reaction at the origin of life.
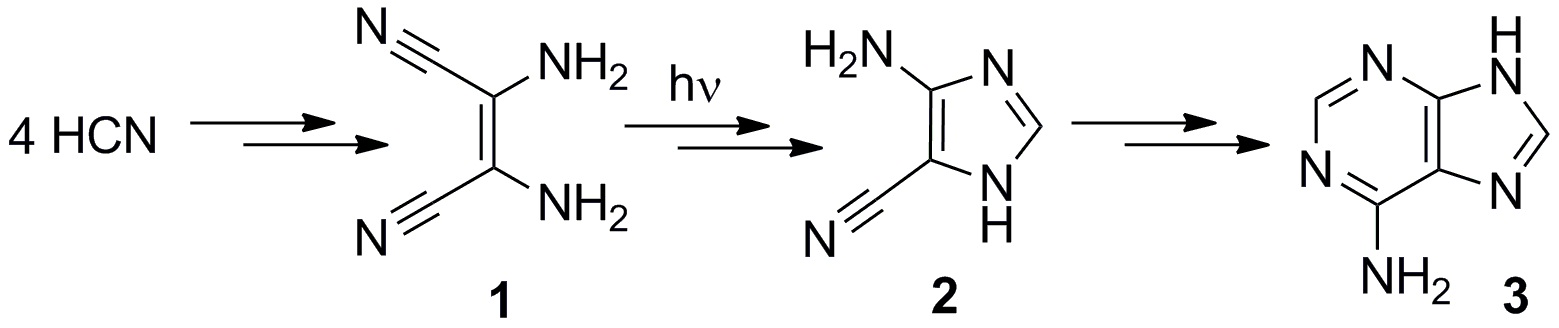
One common aspect in many models of how life appeared on Earth is to suppose that the following photochemical reaction induced by UV radiation was one intermediary step in the synthesis of nucleobases and nucleotides.
In this reaction, HCN polymers (1), which should have existed in prebiotic environments, are converted into imidazole (2).
Although this reaction is known since the 1960’s when it was discovered by Ferris and Orgel, it was still not clear how it happened.
Recently, with my colleagues in Mülheim, Prague and Kahragpur, we could explain the role of the UV radiation for this synthesis.
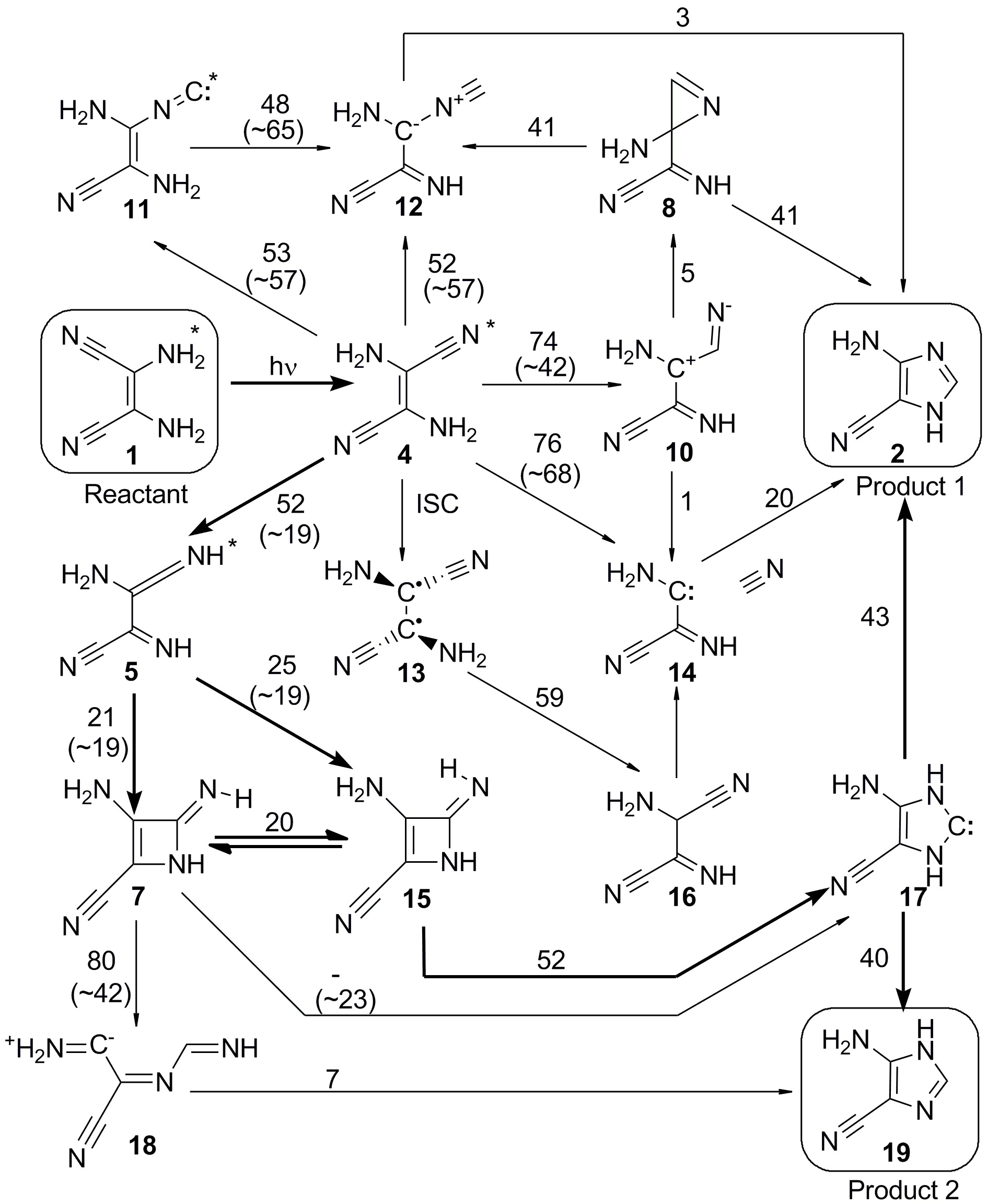
Using computational simulations, first, we mapped many possible reaction pathways. They are shown in the figure below. Like in a puzzle, the reaction (starting in 1 and ending in 2 or 19) can occur by any of those connected arrows.
To complicate a bit, the reaction can occur with the UV energy stored in the electrons (excited state reaction) or with this energy stored in the nuclei (ground state reaction).
To determine which pathway really happens, we computed the energy needed for each step of the reactions. These energies (kcal/mol) are the numbers near the arrows in the figure above. (For excited state reactions they are given between parenthesis.)
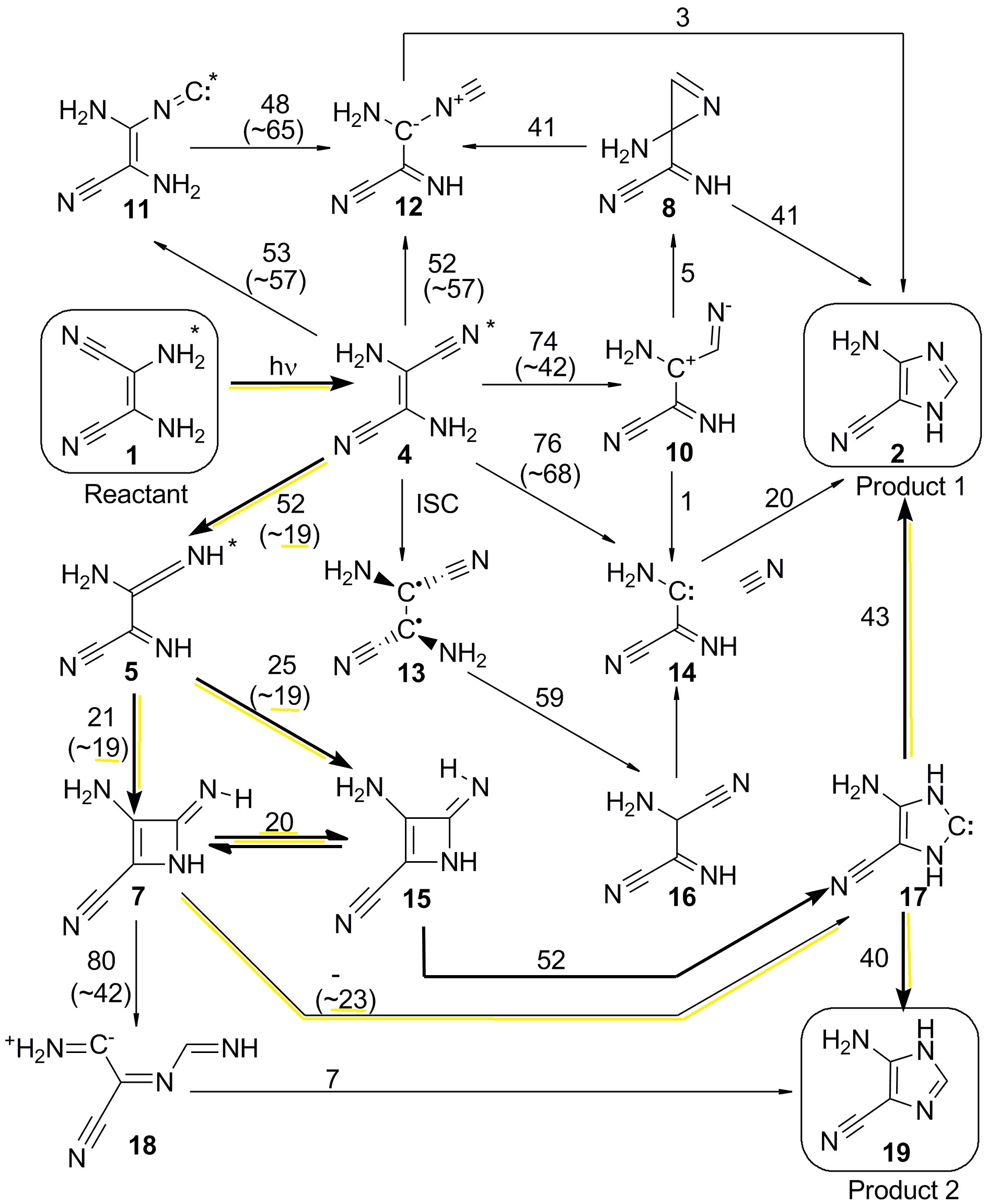
Next, we used molecular dynamics to determine how long it takes to dissipate the UV energy to the environment. The idea is the following: if the energy is in the molecule for a long time, steps with large energy barriers can be overcome. But if the energy is dissipated quickly, only steps with small energy barriers can take place.
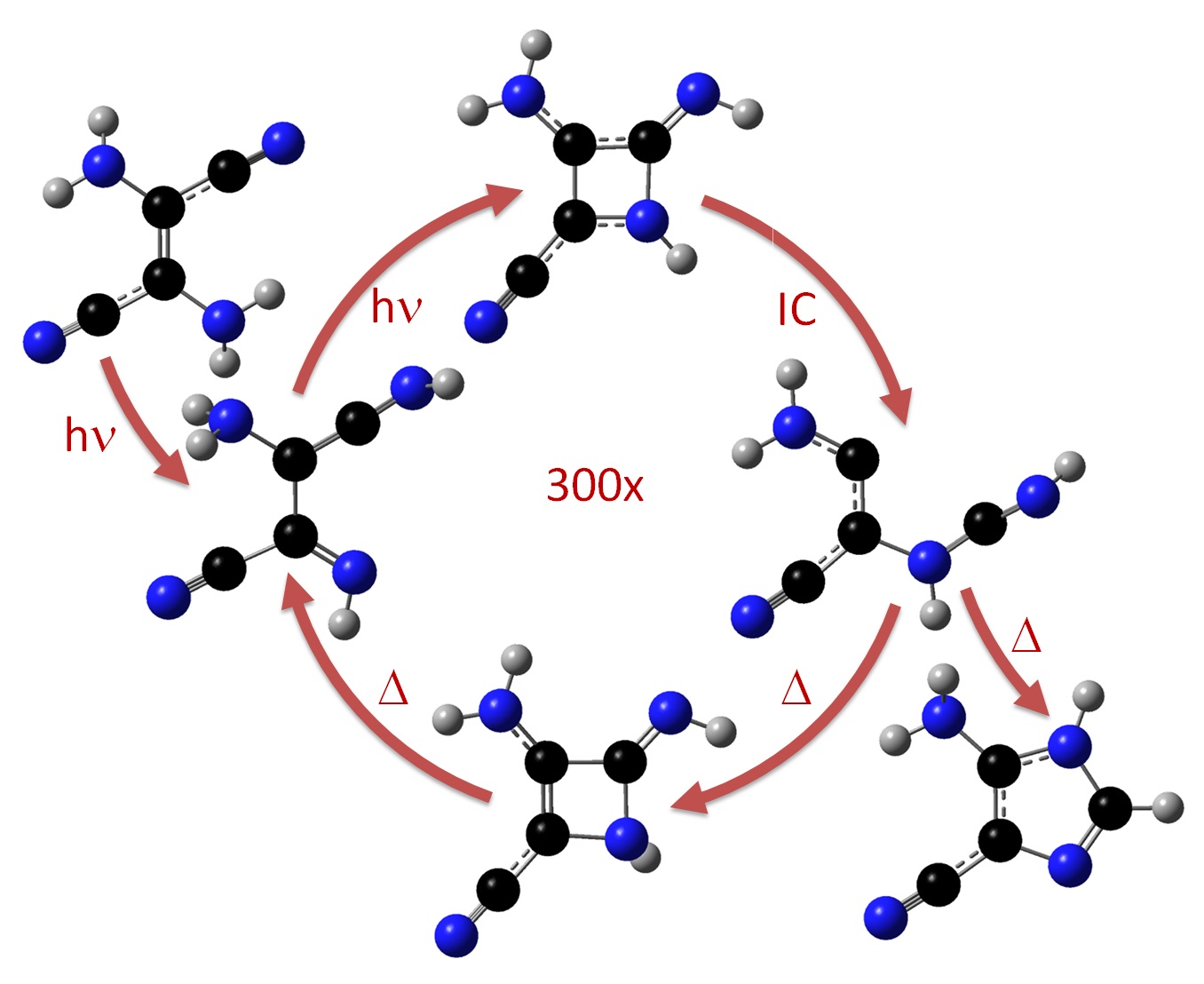
The result of the molecular dynamics together with a chemical kinetics model allowed to establish that no barrier with more than 30 kcal/mol could be overcome. Thus, we could drop most of the pathways and tell that the reaction happens in the steps highlighted in the next figure.
In summary, the reaction happens through a excited-state azetene.
This work was published at the Angewandte Chemie International edition [1].
MB
[1] E. Boulanger, A. Anoop, D. Nachtigallova, W. Thiel, M. Barbatti, Photochemical Steps in Prebiotic Synthesis of Purine Precursors from HCN; Angew. Chem. Int. Ed. 52, 8000 (2013).
doi:10.1002/anie.201303246