How long does it take to transfer energy from a hot molecule to a solvent? We have the answer.
In brief:
- An analytical energy-transfer model is implemented to obtain a chromophore’s heating and cooling times in a given solvent using quantities in nonadiabatic dynamics simulations.
- The model is applied to estimate the energy transfer times of cytosine in argon, benzene, and water.
Photoexcitation instantaneously deposits tens of kcal/mol of energy into a chromophore. On a scale ranging from hundreds of femtoseconds to a few nanoseconds, this energy excess may induce chemical transformations, be reemitted as light, or be dissipated as heat.
In the last case, there are usually two complementary (but sometimes competing) processes. First, the chromophore’s vibrational degrees are heated up after the internal conversion. Second, the heat is transferred to the solvent, cooling down the chromophore.
On which time scales do these heating and cooling processes occur?
Naturally, we expected the answer to this question depends on the solvent: the cooling down is restricted to slow infrared irradiation in the vacuum limit, but it may be much faster if the chromophore is firmly bound to the solvent.
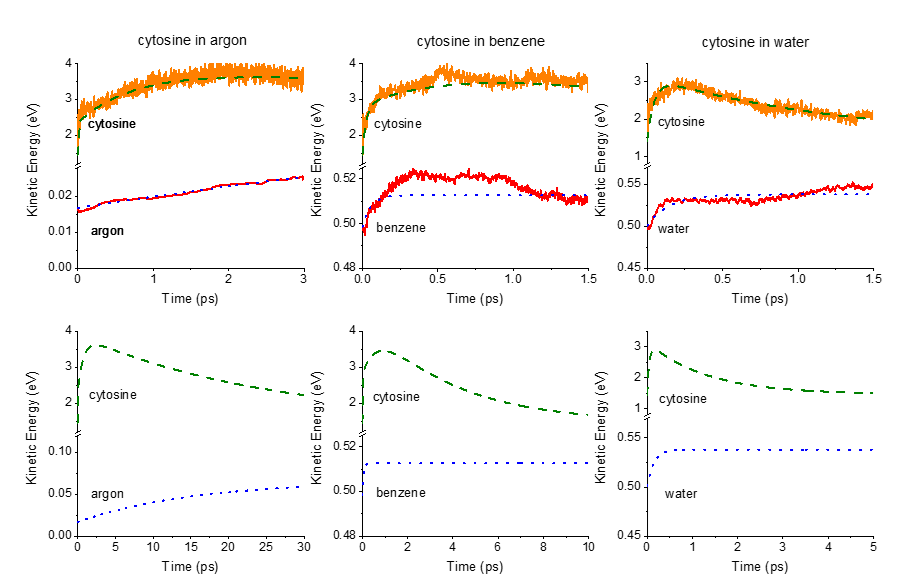
In this work—led by Elizete Ventura and Silmar do Monte—we simulated the nonadiabatic dynamics of cytosine, a prototypical chromophore undergoing ultrafast internal conversion, in three solvents (argon matrix, benzene, and water) spanning an extensive range of interactions.
First, we computed surface hopping dynamics at CASSCF/MM level for cytosine in each solvent. Then, we developed an analytical energy-transfer model to analyze these data and extract heating and cooling times.
The energy transfer model accounts for nonadiabatic effects, excited- and ground-state energy transfer, and can analyze data from any dataset containing kinetic energy as a function of time. It is based on physically motivated equations, parameter constraints, and boundary conditions, compensating for the short simulation times.
Finally, when we fit the QM/MM results with the analytical model, we learn that cytosine heats up on the subpicosecond scale and cools down within:
- 25 ps in argon,
- 4 ps in benzene,
- 1.3 ps water.
The time constants reveal that a significant fraction of the benzene and water heating occurs while cytosine is still electronically excited.
Our initial goal in this work was to estimate the chromophore-to-solvent heat transfer times. These results for cytosine in argon, benzene, and water may be taken as a qualitative indication of the orders of magnitude of the cooling time of other organic chromophores in similar solvents. Nevertheless, the development of the energy-transfer model that can be directly employed with results from any nonadiabatic dynamics simulations goes much beyond that initial goal. It delivers a new protocol to analyze energy transfer in complex environments.
MB
Reference
[1] E. Ventura, S. Andrade do Monte, M. T. do Casal, M. Pinheiro Jr., J. M. Toldo, M. Barbatti, Modeling the heating and cooling of a chromophore after photoexcitation, Phys. Chem. Chem. Phys. (2022). DOI: 10.1039/D2CP00686C