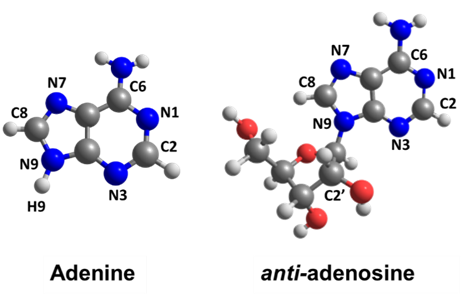
Intramolecular energy transfer at low temperatures delays adenosine’s internal conversion.
In brief:
- While the internal conversion time of adenine does not depend on temperature, adenosine’s lifetime strongly does.
- Our surface hopping simulations at 0 and 400 K revealed that the reason is vibrational energy transfer to the ribose group in adenosine.
- At 0 K, this transfer reduces the energy available to reach the conical intersection so much that the internal conversion is delayed.
In chemistry, understanding molecular behavior in different environments and conditions is a never-ending quest. One such fascinating exploration has recently revealed some intriguing insights into the behavior of adenine and adenosine, two essential molecules in biochemistry.
Researchers have long been curious about how temperature affects the excited-state lifetime of these molecules. Excited-state lifetime is a crucial parameter in chemistry, as it can determine the outcome of various chemical reactions and processes. In a groundbreaking study, we have shed light on this enigma, providing valuable insights into the temperature-dependent behavior of adenine and adenosine.
The Exciting Quest
The journey begins with a simple yet profound question: Does temperature influence the excited-state lifetime of adenine and adenosine? To answer this question, West and co-workers embarked on an experiment that involved simulating the dynamics of these molecules in different temperatures.
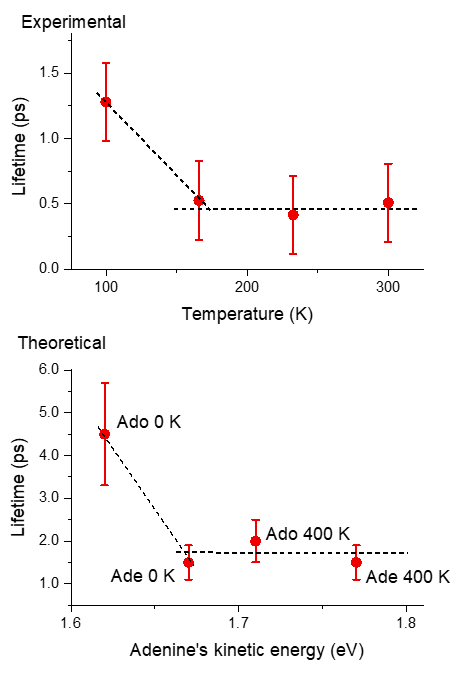
At the heart of their investigation lies the concept of excited-state lifetime. When a molecule absorbs energy and enters an excited state, it eventually returns to its ground state. The time it takes for this transition is known as the excited-state lifetime. It was found that this lifetime remained constant for adenine, irrespective of temperature. In contrast, adenosine’s lifetime was found to be highly temperature-dependent.
The Temperature Puzzle
The real intrigue in this study lies in explaining why adenosine’s excited-state lifetime changes with temperature while adenine’s lifetime remains unaltered. The puzzle is further complicated because the temperature dependence observed for adenosine does not follow the conventional Arrhenius behavior commonly seen in chemical reactions.
The Solution: Intramolecular Vibrational Energy Transfer
Surface hopping simulations provided a breakthrough in understanding this phenomenon. We incorporated temperature effects into the initial conditions generated via a Wigner sampling with thermal corrections. Then, we ran dynamics simulations for photoexcited adenine and adenosine in the gas phase at 0 and 400 K. Through this meticulous approach, we discovered that adenosine’s temperature-dependent behavior arises from intramolecular vibrational energy transfer.
In simpler terms, what happens is that the energy from the excited state of adenosine is efficiently transferred from the nucleobase to the ribose group. At 400 K, although this process reduces the mean kinetic energy of the adenine moiety, enough energy is left to allow fast internal conversion. At 0 K, however, adenosine cannot rely on a supply of thermal energy. Thus, the kinetic energy drops to a point that inhibits internal conversion. As a result, adenosine’s excited-state lifetime at 0 K is longer than at 400 K by a factor of 2.3.
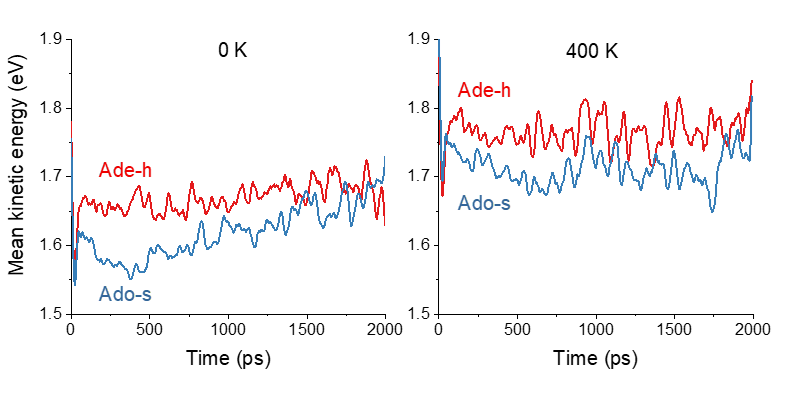
In turn, adenine has no chemical group to transfer the excess energy. Its internal conversion always occurs with the same speed, independently of temperature.
This elegant explanation clarifies why adenosine behaves differently from adenine regarding temperature dependence. The intricate dance of intramolecular energy transfer is key to understanding this phenomenon.
Energy transfer of viscosity?
Another important revelation from our study is the dismissal of an alternative explanation that was previously proposed—viscosity. West and his colleagues suggested that differences in viscosity at different temperatures could be responsible for the observed variations in excited-state lifetime. Their main motivation for this explanation was the non-Arrhenius behavior of adenosine lifetime (see bottom figure aside).
However, our simulations definitively ruled out this influence. We showed that energy transfer alone can predict the same behavior (top figure aside).
The Takeaway
In the quest to understand the behavior of photo-excited molecules, especially in the context of temperature, this study on adenine and adenosine provides valuable insights. It demonstrates energy transfer within parts of the molecule can profoundly affect the photodynamics.
Moreover, it showcases the power of carefully crafted simulation techniques in unraveling complex chemical phenomena.
MB
Reference
[1] R. Mansour, J. M. Toldo, S. Mukherjee, M. Pinheiro Jr, M. Barbatti, Temperature effects on the internal conversion of excited adenine and adenosine, Phys. Chem. Chem. Phys. (2023). DOI: 10.1039/D3CP03234E