The surprising chemistry of resveratrol reveals its hidden potential to produce singlet oxygen.
In brief:
-
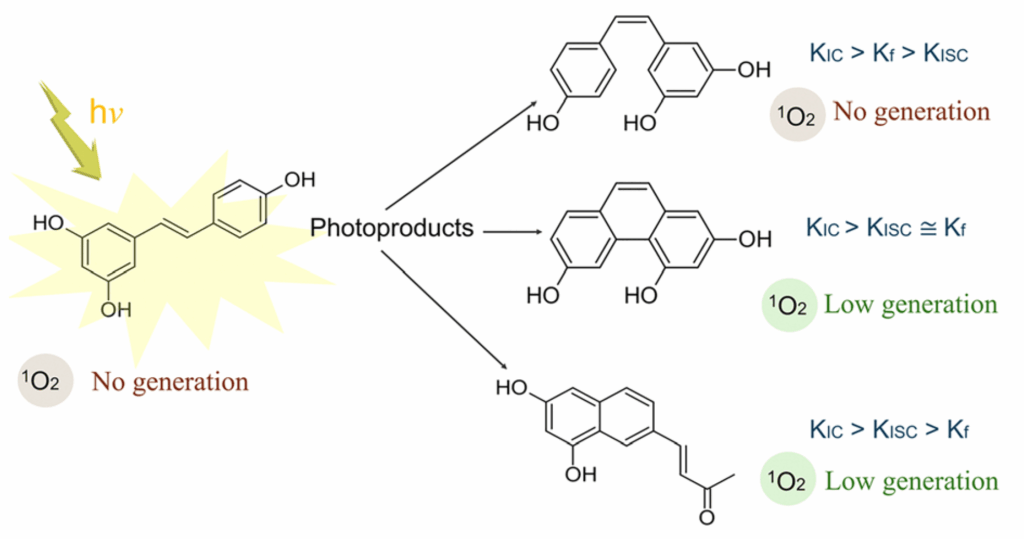
Resveratrol transforms into new molecules when exposed to UV radiation. Some may create reactive singlet oxygen.
-
Our theoretical study explains which resveratrol derivatives are responsible for singlet oxygen generation.
- THP and resveratrone are the main suspects, though with low quantum yields.
Resveratrol is usually celebrated as an antioxidant, found abundantly in grapes, red wine, and peanuts. It’s famous for its potential health benefits, from anti-aging properties to protecting our bodies from oxidative stress. But resveratrol has a lesser-known, more reactive side: under UV light, it transforms can generate singlet oxygen—a highly reactive species.
This phenomenon had been observed in experiments before, but we set out to understand the how and why using theoretical chemistry. In our latest study, led by Mariana Yoshinaga, we evaluated the potential of several resveratrol derivatives to yield singlet oxygen. Two standout derivatives are THP (2,4,6-trihydroxy-phenanthrene) and resveratrone.
Singlet oxygen isn’t just a reactive species—it’s also a useful one. In medicine, it’s harnessed in photodynamic therapy (PDT) to treat cancer and skin diseases, by selectively targeting harmful cells.
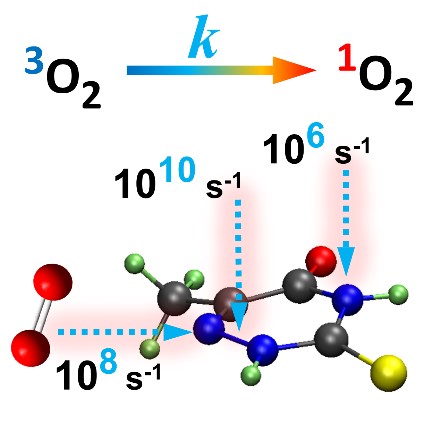
Using quantum chemical calculations, we examined how efficiently trans-resveratrol, cis-resveratrol, THP, and resveratrone absorb UV light, how they populate triplet excited states (a key step to form singlet oxygen), and whether they can transfer energy to molecular oxygen. The results were clear: both THP and resveratrone can, under the right conditions, produce singlet oxygen. They may not do it in large quantities—but enough to matter.
This finding helps clarify the photochemistry of resveratrol and points toward potential medical applications for its derivatives. With small tweaks to the molecular structure, one could imagine optimizing these molecules as more efficient photosensitizers.
So no, your glass of wine won’t start a singlet oxygen riot in your bloodstream. But in a lab or clinical setting, the same chemistry could be turned into something powerful and therapeutic.
MB
Reference
[1] M. Yoshinaga, J. M. Toldo, W. R. Rocha, M. Barbatti, Photophysics of Resveratrol Derivatives for Singlet Oxygen Formation, Phys. Chem. Chem. Phys. (2025).10.1039/D5CP00840A