Excited-state lifetime is deeply affected by the conical intersection topography.
In brief:
- Time-resolved spectroscopy of ethyl sinapate (ES) in the gas phase tells that its internal conversion to the ground state occurs within 4.5 ps. Similar measurements of diethyl sinapate (DES) reveal a 0.3 ps lifetime.
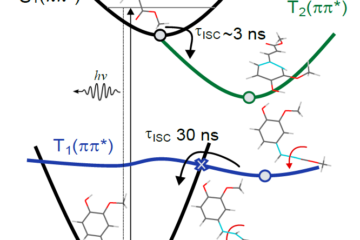
- Computational modeling shows this difference is due to the conical intersection topography, sloped for ES and peaked for DES.
Have you ever wondered how some materials can convert sunlight into heat with incredible efficiency? Well, the world of chemistry has been hard at work unraveling the secrets behind these photothermal materials, and the results are nothing short of fascinating.
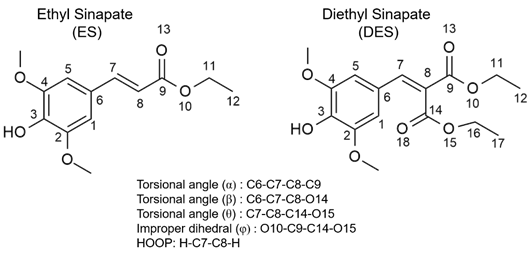
In a recent experimental-theoretical collaboration with Stavro’s team, Josene Toldo and I have delved deep into the molecular structure of two biomimetic systems, ethyl sinapate (ES) and its symmetrical counterpart, diethyl sinapate (DES), to understand the magic behind their ability to capture and convert sunlight into heat.
Let’s break down the science behind this intriguing discovery.
First, meet our star molecules: ES and DES. These compounds are part of a family of chemicals known as sinapate esters. They’re not naturally occurring; they’re synthetic compounds designed to mimic certain aspects of natural processes, such as photosynthesis.
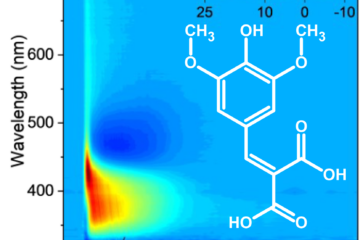
Now, let’s talk about what happens when these molecules encounter sunlight. When exposed to light, both ES and DES get excited. Here’s where things get really interesting. After the light-induced excitement, these molecules want to return to their ground state. Our time-resolved spectra showed that DES, the symmetrical one, does this at an astonishing speed, 0.3 ps, more than ten times faster than ES, its non-symmetrical sibling, which converts within 4.5 ps.
So, what’s the secret behind DES’s lightning-fast return to the ground state?
The answer lies in the potential energy surfaces and conical intersection topography, which might sound like complex terms but bear with us. Imagine these surfaces as landscapes where the molecules can roam. DES seems to have discovered a shortcut, a peaked conical intersection, on its way back to the ground state. ES, however, returns to the ground state through a slipped intersection, which is less efficient and elongates the lifetime.
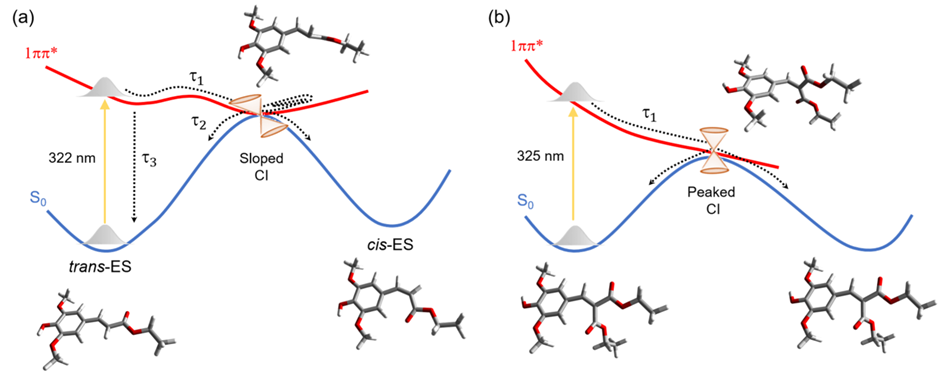
So, why does all of this matter? Well, it turns out that how these molecules return to the ground state is crucial for their photothermal properties. In simpler terms, the faster they can return to their ground state after absorbing sunlight, the more efficient they are at converting that sunlight into heat without breaking into secondary chemicals.
And that’s exactly what photothermal materials are all about – harnessing sunlight’s energy without undergoing chemical transformations. Understanding the role of symmetry in this process, as shown in the case of DES, provides a blueprint for designing even more efficient photothermal materials in the future.
MB
Reference
[1] J. Dalton, J. M. Toldo, F. Allais, M. Barbatti, V. G. Stavros, Understanding the Impact of Symmetrical Substitution on the Photodynamics of Sinapate Esters Using Gas-Phase Ultrafast Spectroscopy, J. Phys. Chem. Letters (2023). DOI: 10.1021/acs.jpclett.3c02134