Energetic stability of doubly-excited states depends on the number of π electrons.
In brief:
- The relative stability of dark (doubly-excited) and bright (singly-excited) states in thiophene-DPP oligomers is controlled by the π scaffold.
- The electronic structure of these molecules behaves analogously to those of polyenes.
- Their acceptor-donor features play no role in the bright/dark stability.
Diketopyrrolopyrrole (DPP) is a pivotal functional group in tuning the physicochemical properties of novel organic photoelectronic materials.
Motivated by the design of new intramolecular singlet-fission materials, Mukhopadhyay et al. proposed a combination of a thiophene-DPP derivative (with charge-transfer (CT) character) with a vinyl linker (polyene character) (Figure 1b).
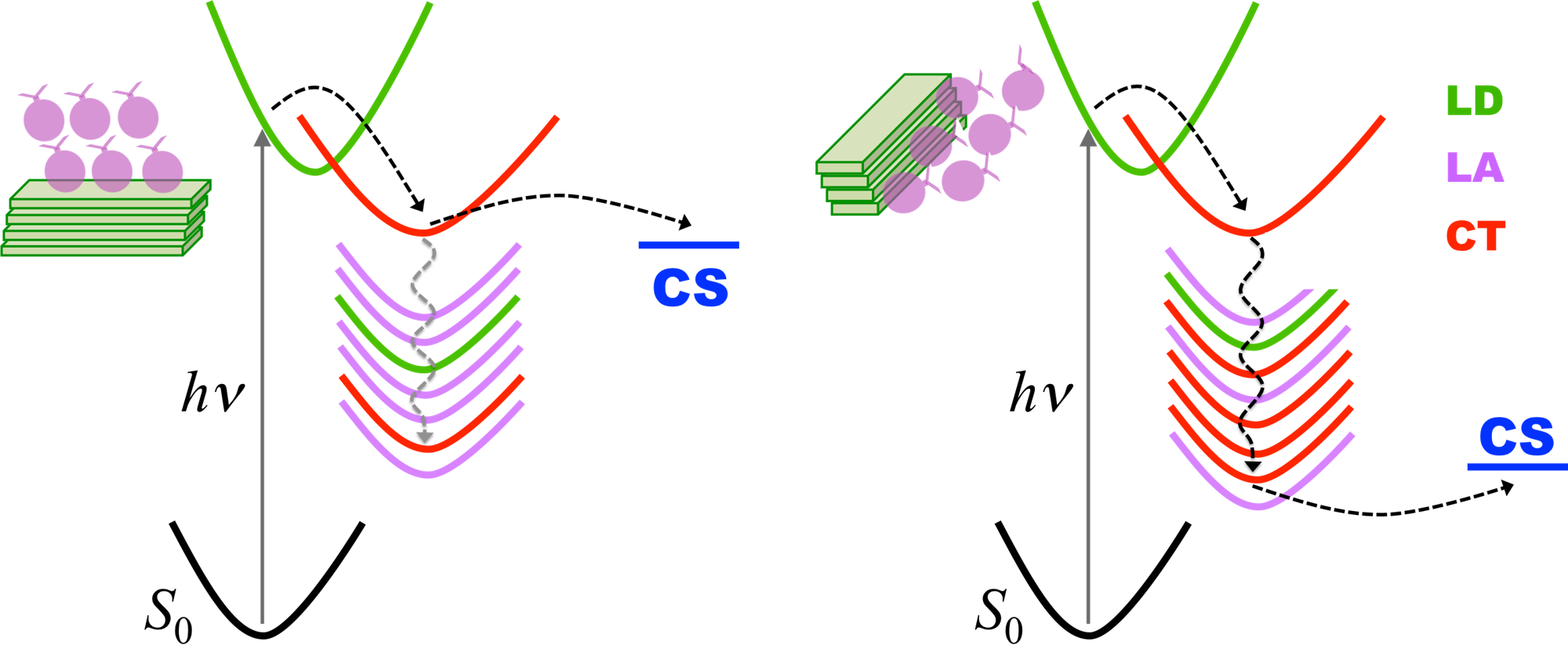
They didn’t observe singlet fission in this new material. However, they did detect fast internal conversion from the initial bright state into a short-lived dark singlet state, with no evidence of substantial conformational change, triplet formation, or charge separation. This internal conversion in the dimer (Figure 1b) is remarkable, considering that the monomer (Figure 1d) is fluorescent.
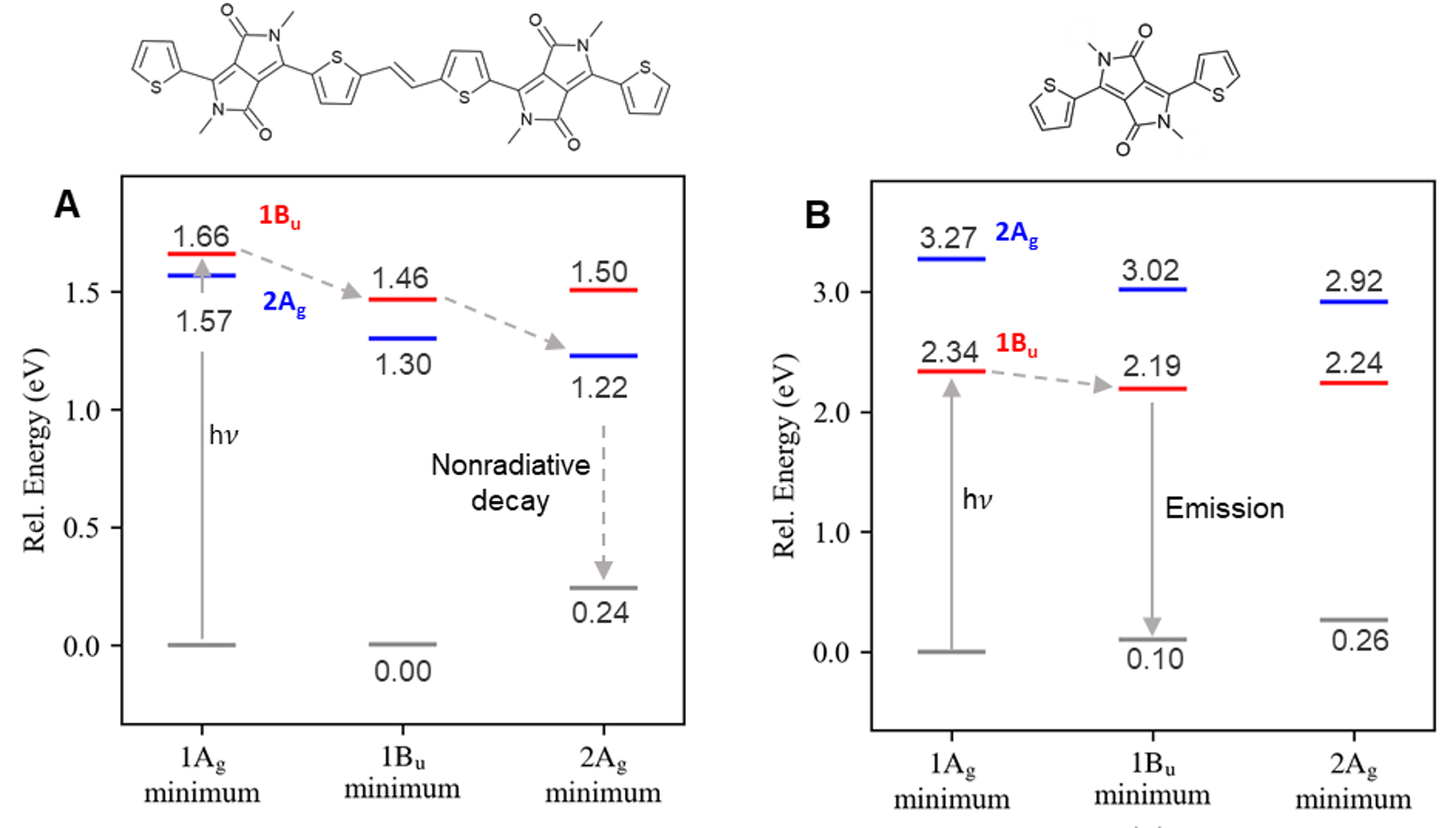
We explained this fluorescence quenching using computational chemistry. The DPP-thiophene dimer (Figure 2A) has a low-lying doubly excited state (2Ag) that is not energetically accessible to the monomer (Figure 2B). This state delays the fluorescence allowing internal conversion to occur first. [See Box 1 for a discussion on computational methods.]
At this point, we know that the fluorescence quenching is caused by the dark/bright state inversion between the monomer and dimer. However, why do they invert? Why is the doubly-excited state so stable in the dimer?
There are two possible causes:
- The acceptor/donor character of the sub-units in the dimer.
- The large number of π electrons in the dimer.
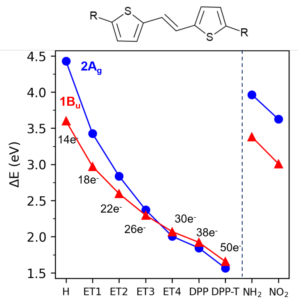
We characterized the doubly-excited state wavefunction by systematically changing the derivatives to tune the π-scaffold size (Figure 1f-j) and the acceptor and donor characters (Figure 1k-l).
The origin of this state’s stabilization is related to the increase in the π-system and not to the charge-transfer features. Note in Figure 3 how the number of π electrons stabilizes the doubly-excited state while acceptor/donor groups don’t help much.
This analysis delivers core conceptual information on the electronic properties of organic chromophores arranged symmetrically around a vinyl linker, opening new ways to control the balance between luminescence and internal conversion.
These theoretical results are published in Ref. [1]. They are also used in a companying paper (Ref. [2]) to rationalize many features in the experimental spectra recorded by our colleagues in Bristol, led by Tom Oliver.
MB
References
[1] M. T. do Casal, J. M. Toldo, F. Plasser, M. Barbatti. Using diketopyrrolopyrroles to stabilize double excitation and control internal conversion, Phys. Chem. Chem. Phys. (2022). DOI: 10.1039/D2CP03533B
[2] D. Polak, M. Telles do Casal, J. M. Toldo, X. Hu, G. Amoruso, O. Pomeranc, M. Heeney, M. Barbatti, M. N. R. Ashfold, T. Oliver. Probing the electronic structure and photophysics of thiophene–diketopyrrolopyrrole derivatives in solution, Phys. Chem. Chem. Phys. (2022). DOI: 10.1039/D2CP03238D
0 Comments