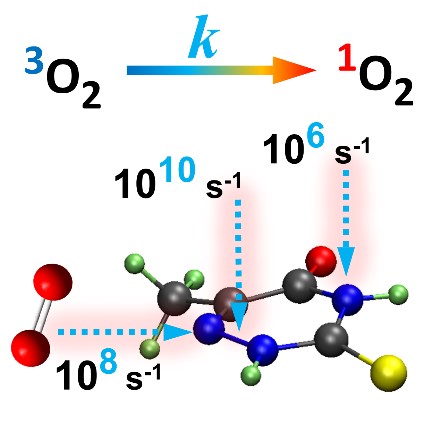
Functionalization of the sugar group can be used to control the triplet-decay rate of thionucleosides.
In brief:
- Presence of sugar enhances the triplet decay in thiopyrimidines but not in thiopurines.
- This effect is controlled by steric interactions between the sugar group and the sulfur atom.
- This discovery opens a new way to design compounds with a controlled triplet-decay rate.
The decay of the triplet state of photosensitizers is essential to their performance for singlet-oxygen generation. Experiments have shown that in thionucleosides this decay is enhanced compared to that of the corresponding thionucleobases. Nevertheless—as you can see in the table below—this enhancement is much stronger for pyrimidines (by factor 14) than for purines (by factor 2).
| Triplet decay rate ratio thionucleoside/thionucleobase |
||
| Theory (our work) |
Experimental (literature) |
|
| 2-thiothymidine | 18 | 14 ± 2 |
| 6-thioguanosine | 1 | 2.0 ± 0.3 |
| 1’-methyl-2-thiothymidine | 243 | – |
In this project led by Shuming Bai, we applied quantum-chemical methods and chemical-kinetic modeling to investigate the effects of sugar substituent on the triplet decay of thionucleosides. To compute the triplet decay rates, we used a modified Marcus approach, given by
where spin-orbit coupling , reorganization energy
, and activation energy
are obtained from the reaction pathway connectinng the triplet minimum to the triplet-singlet crossing.
The computed rates for the energetically favored conformers of thiothymidine, thiouridine, and thioguanosine (and the respective thionucleobases) show a remarkable absolute quantitative agreement with the experimental results. (Some of the relative results are given in the table above.)
We additionally show that the triplet decay enhancement is caused by the repulsion interaction between the sugar group and the sulfur atom, which reduces the activation energy for intersystem crossing, by destabilizing the T1 minimum. In some instances, an intramolecular hydrogen bond stabilizes the energy of the T1/S0 crossing point, also reducing the activation energy.
This sugar-sulfur interaction, indeed, also explains why the effect is stronger in pyrimidines than in purines: the sulfur atom is much closer to the sugar group in pyrimidines.
This molecular understanding of the mechanism of enhanced triplet decay provides a guideline to control the triplet decay rate, which was computationally tested in new thiothymidine derivatives. For instance, we designed a new compound — the 1’-methyl-2-thiothymidine — to maximize these steric interactions, expecting that it would have a much large triplet decay rate than any of the molecules studied before. And, in fact, our calculations predict a ratio of 243 between its rate and that of thiothymine.
These results have been published in Ref. [1].
MB
Reference
[1] Shuming Bai and Mario Barbatti, Mechanism of Enhanced Triplet Decay of Thionucleobase by Glycosylation and Rate-Modulating Strategies, Phys. Chem. Chem. Phys. DOI: 10.1039/C8CP02306A (2018).