CH∙∙∙Cl hydrogen bond in chloromethane may be formed by UV irradiation.
CH3Cl is one of the main sources of atomic Cl in the high atmosphere. And this is relevant because this atomic Cl attacks the ozone layer.
Although the lowest Cl dissociation channel of CH3Cl is well-known, there is almost nothing in the literature about the other channels, which are accessed via UV photoexcitation.
Together with my colleagues from the Federal University of João Pessoa and from the Rural Federal University of Rio de Janeiro, we have been investigating these reaction paths [1].
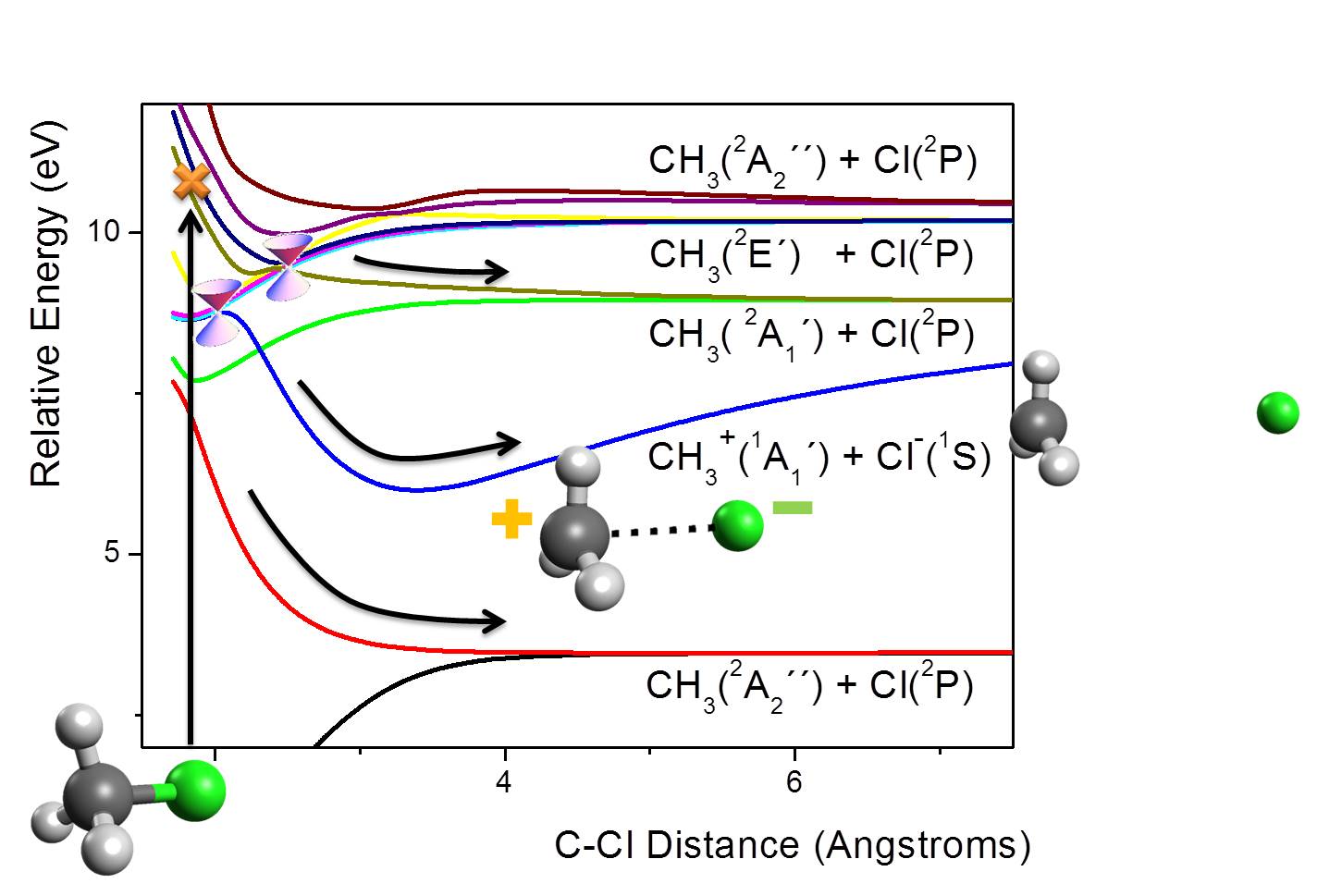
This was a hard-core quantum-chemical study based on multireference configuration interaction (MRCI) method. The main results are shown in the figure below, illustrating five dissociation channels.
When CH3Cl reaches the stratosphere, it may be excited by far-UV radiation. After that, a cascade of nonadiabatic processes takes place, leading to a fast relaxation through the manifold of excited states. During this relaxation, at least three deactivation channels bellow 10 eV may be populated.
Even though the relaxation process is complicated by the large number of states involved, we have shown that there are two specific C-Cl distances with multiple conical intersections. They work as hot-spots for nonadiabatic transitions.
Among the dissociation channels, there is one in particular resulting in charged fragments: CH3+ and Cl–. Due to the Coulombic attraction between these fragments, the may form a strongly bound electrostatic complex, CH3+∙∙∙Cl–.
We have found out that the CH3+∙∙∙Cl– electrostatic complex can be further stabilized by almost 1 eV, by a relative rotation of the fragments, forming a H2CH+∙∙∙Cl– complex. The analysis of this complex shows that this additional stabilization is due to an underlying CH∙∙∙Cl hydrogen bond.
CH∙∙∙Cl hydrogen bonds are common in solid state, where they are induced by crystal constraints. However, this is the first time that such a bond is reported for a small molecule in the gas phase. In the paper [1], we propose a time-resolved three photons experimental set-up to detect this species.
Although the main interest of these findings concerns basic research, to know that CH∙∙∙Cl hydrogen bonds may be formed induced by UV radiation may in the future lead to the chemical design of new organic photovoltaic materials.
Reference
[1] V. C. de Medeiros, R. B. de Andrade, E. F. V. Leitão, E. Ventura, G. F. Bauerfeldt, M. Barbatti, S. A. do Monte, Photochemistry of CH3Cl: Dissociation and CH∙∙∙Cl hydrogen bond formation, J. Am. Chem. Soc., doi:10.1021/jacs.5b10573 (2015). Free versions of the paper are available in this link.


1 Comment
Chlorine elimination in HCFC-133a – Light and Molecules · March 6, 2019 at 12:26 PM
[…] which also has a great appeal to fundamental research due to the transient formation of a rare CH∙∙∙Cl bonded moiety. This research has been done employing state-of-the-art multireference methods and mixed […]
Comments are closed.