Synthesis and characterization of organic materials with high electron mobility.
Direct arylation is emerging as a highly promising method to construct inexpensive conjugated materials for large area electronics from simple and environmentally benign building blocks. Our experimental colleagues, led by Michael Sommer from the University of Freiburg, showed that exclusive α-C-H selectivity is feasible in the DA of π-extended monomers having unsubstituted thiophene or furan units, leading to fully linear materials.
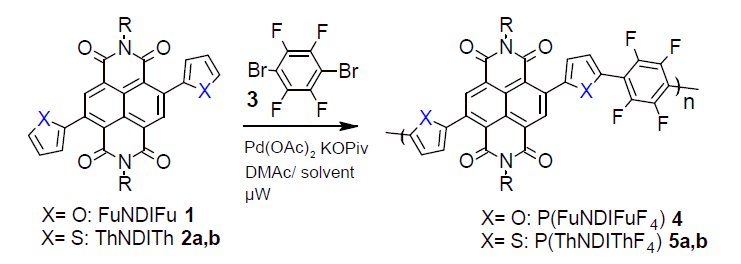 Two new naphthalene diimide-based conjugated copolymers P(FuNDIFuF4) and P(ThNDIThF4) were synthesized. These are highly active in the visible region and have high charge mobility, make them good candidates to be used in organic photovoltaic devices.
Two new naphthalene diimide-based conjugated copolymers P(FuNDIFuF4) and P(ThNDIThF4) were synthesized. These are highly active in the visible region and have high charge mobility, make them good candidates to be used in organic photovoltaic devices.
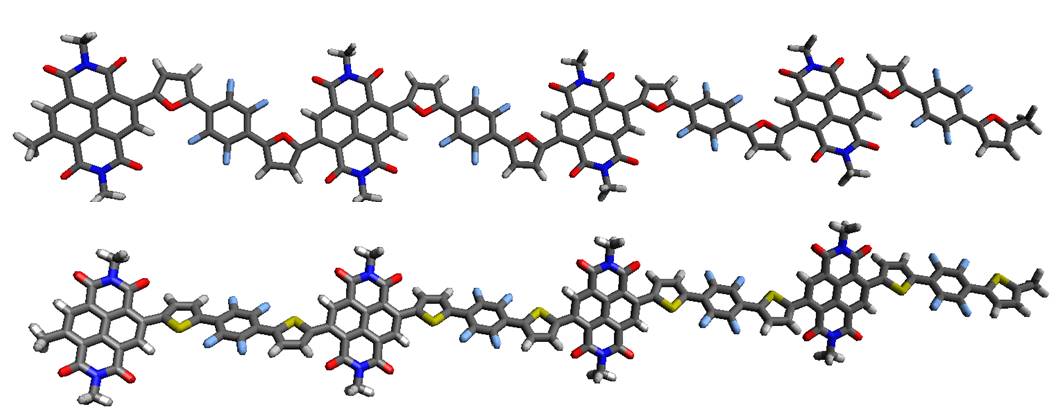 Daniele Fazzi, Walter Thiel, and I jointed this tour de force involving also researchers from Italy and Australia to characterize these new materials using diverse experimental and computational techniques.
Daniele Fazzi, Walter Thiel, and I jointed this tour de force involving also researchers from Italy and Australia to characterize these new materials using diverse experimental and computational techniques.
- A. Luzio, D. Fazzi, F. Nübling, R. Matsidik, A. Straub, H. Komber, E. Giussani, S. Watkins, M. Barbatti, W. Thiel, E. Gann, L. Thomsen, C. McNeill, M. Caironi, M. Sommer, Structure-function relationships of high-electron mobility naphthalene diimide copolymers prepared by direct arylation; Chem. Mater., doi:10.1021/cm503033j (2014). doi:10.1021/cm503033j