Paper explains the absorption spectrum of guanidine and guanidinium.
Guanidine and its protonated form (guanidinium) are compounds of great importance for chemistry and biology. Curiously, there is almost no information about their spectroscopic properties in the literature.
 Ivana Antol and Zoran Glasovac from the Ruđer Bošković Institute in Zagreb, in collaboration with our group, took the challenge of computing and assigning the UV spectra of these molecules.
Ivana Antol and Zoran Glasovac from the Ruđer Bošković Institute in Zagreb, in collaboration with our group, took the challenge of computing and assigning the UV spectra of these molecules.
Based on CASPT2 optimizations and energy calculations, we have been able to determine excitation spectra, ionization potentials, and internal conversion pathways for both molecules.
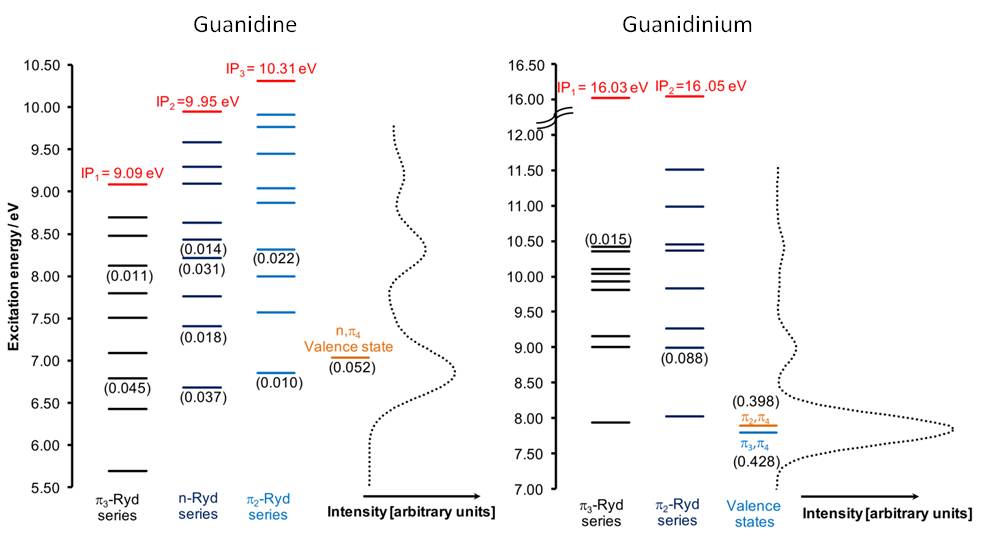
As show in the figure below, the vertical excitation spectrum of the neutral and cation species are completely different. While guanidine has the onset at 6 eV and the weak first band mixes valence nπ* and Rydberg states, protonated guanidine has the onset at 7 eV and strongly absorbs through a ππ* excitation.
A particularly interesting aspect of the absorption spectrum of guanidine is that the ππ* excitation is absent, at least up to the ionization threshold.
Reference
I. Antol, Z. Glasovac, R. Crespo-Otero, M. Barbatti, Guanidine and guanidinium cation in the excited state – theoretical investigation; J. Chem. Phys. doi:10.1063/1.4892569 (2014)
1 Comment
Guanidinium: A New Analytical Tool to Detect Anions | Light and Molecules · September 1, 2016 at 1:53 PM
[…] This work was recently published at the Journal of Physical Chemistry A [1]. For more information on the excited states of guanidine and guanidinium alone, check this post. […]
Comments are closed.