Looking at the future: organic electronics
Donor-acceptor organic heterojunctions (DA-OHJ) have been extensively explored for photodevices. They are promising materials due to their potential low-cost and plastic properties.
The search for new DA-OHJ goes through the cumbersome process of designing, synthesizing and testing new molecular complexes, heuristically improving certain properties over the previous generation of complexes.
Computational simulations may be a valuable tool for this search, as they may help to
- Explain the basic photophysical processes;
- Pre-screen hypothetical molecular complexes prior the synthesis.
To achieve these aims, however, it is needed to
- Assess the quality of the computational predictions;
- Develop new research protocols to deal with such complex systems;
- Connect atomistic simulations to mesoscopic simulations.
Our research group has systematically tackling each of these points in a long-term project.
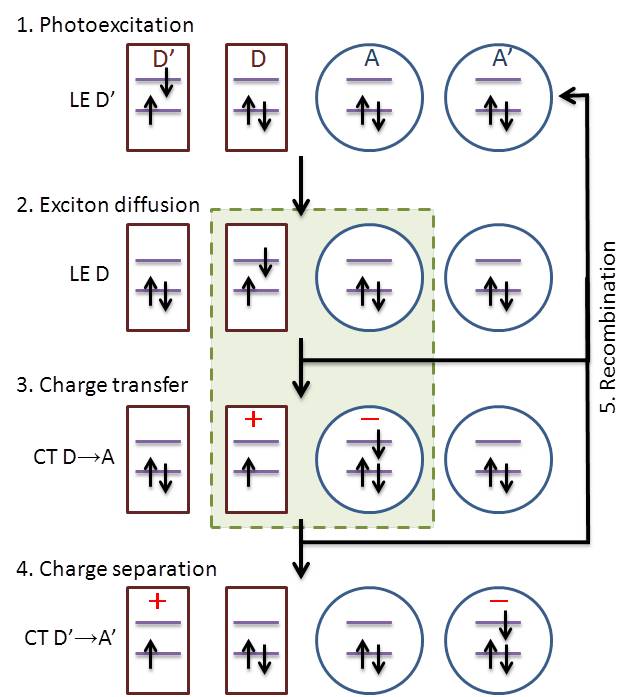
Photophysics of a D-A-OHJ
Conceptually, the operation of a DA-OHJ is based on the 5 steps above.
How accurate is this picture? How does exactly each step take place?
These are questions that we are addressing now.
In a series of investigations, we have learned:
- How charge recombination in squaraine-fullerene complexes follows a non-Marcus regime
- How the ultrafast relaxation leads to exciton localization in oligothiophenes
- How to functionalize an oligothiophene to boost charge transfer
- How to orient oligothiophenes and thiophenes to maximize hot and cold excitons
- How the absorption and fluorescence spectra of PPVs depends on the number of oligomers
- How the oxidation state can control the luminescence of sulfur-bridged naphthalene dimers
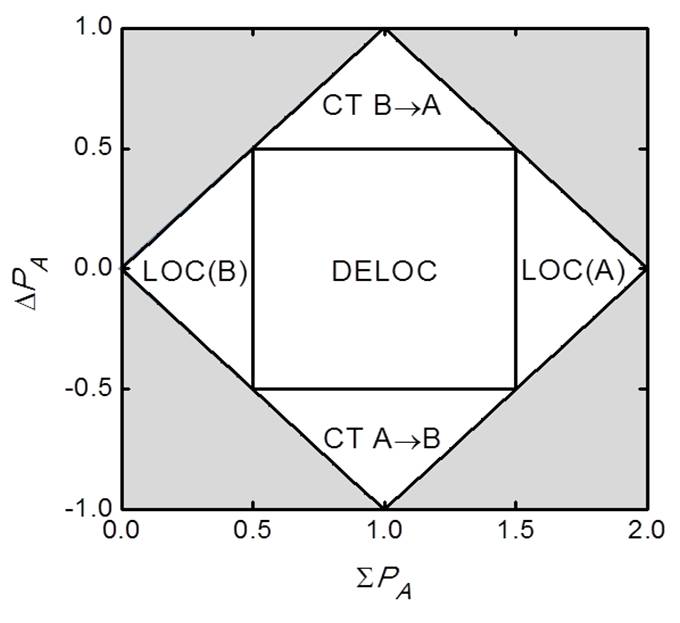
Automatic State Classification
Based on a CI approximation for the excited-state densities, states of a D-A complex can be classified as localized, delocalized or charge-transfer (Ref).
We have applied this approach to analyze the electronic structure of thiophene oligomers (Ref) and squaraine dies (Ref) in complexes with fullerenes.
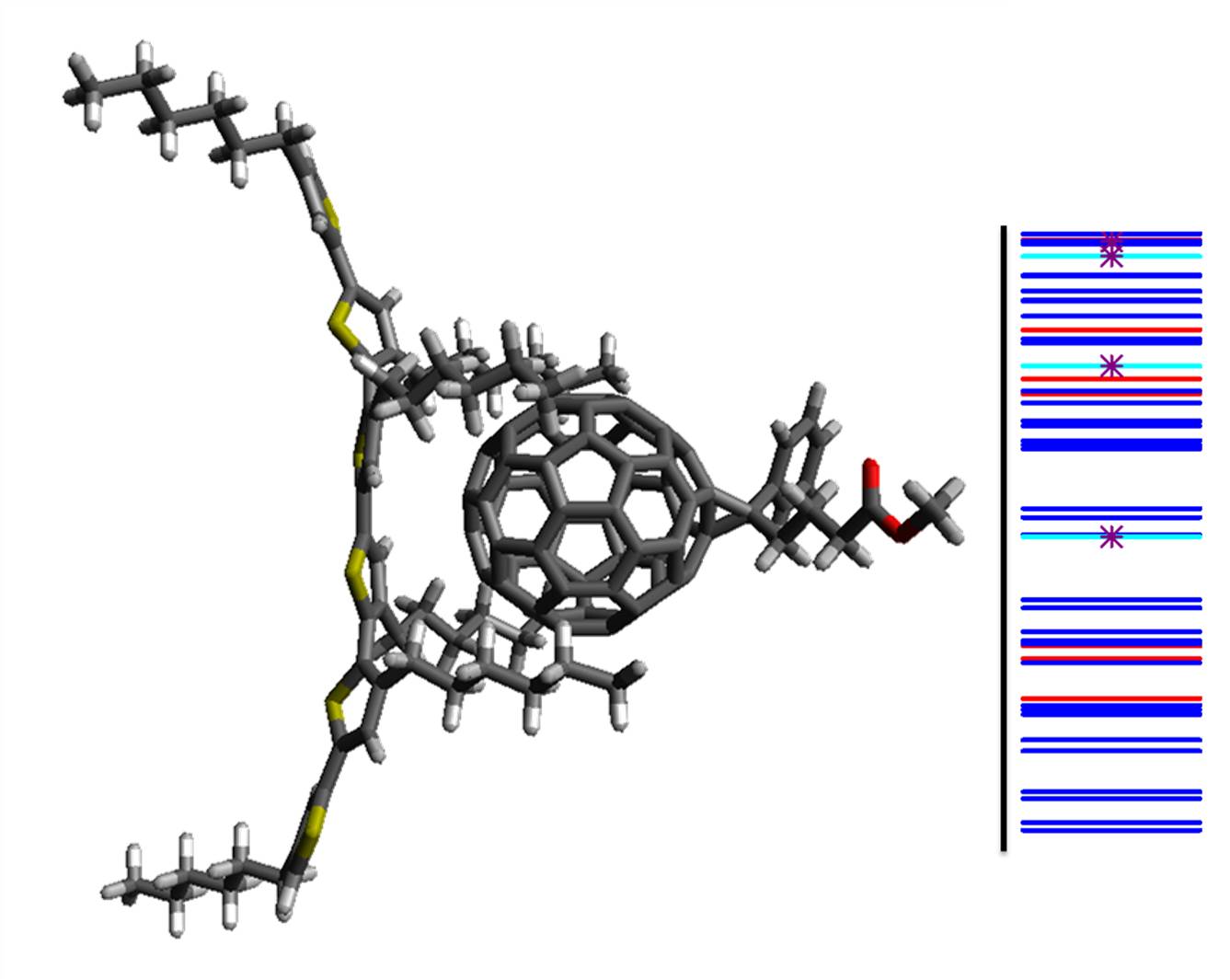
Electronic structure of P3TH-PCBM hetero dimer. Blue: LOC(PCBM); Cyan: LOC(P3TH); Red: CT(P3TH-PCBM). Starts indicate bright states.