Hint: it isn’t only about SOC!
In brief:
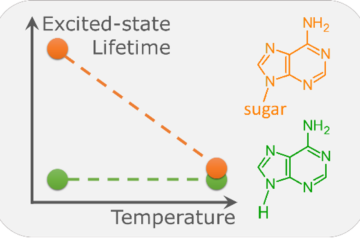
- The study investigates the contrasting T1 lifetimes of 6-thioguanine (6tGua) and 6-selenoguanine (6SeGua), which is crucial for photosensitizer efficiency.
- Findings reveal a solvent-mediated mechanism wherein a hydrogen bond with water significantly prolongs the T1 lifetime of 6tGua.
- Insights underscore the pivotal role of solvents in shaping the dynamics of excited states, offering implications for photosensitizer applications in aqueous environments.
Photosensitizers, molecules that absorb light and transfer energy to surrounding molecules, play a crucial role in diverse applications ranging from photodynamic therapy to solar energy conversion. A recent study led by Shuming Bai (Chinese Academy of Sciences) delves into the intriguing dynamics of two structurally similar molecules—6-thioguanine (6tGua) and 6-selenium guanine (6SeGua)—shedding light on their disparate lifetimes in the triplet state, crucial for their efficacy as photosensitizers.
The decay of the T1 state to the ground state is a fundamental aspect governing the lifetime of excited states and, consequently, the temporal window for sensitization. Traditionally, sulfur and selenium substitutions in carbonyl groups have been recognized for their ability to red-shift absorption spectra and enhance triplet yield, owing to their large spin-orbit coupling (SOC). This modification transforms nucleobases into promising candidates for photosensitizer applications across various domains.
However, a notable puzzle arises when comparing the T1 lifetimes of 6tGua and 6SeGua. Experimental investigations have revealed that the triplet decay rate of 6SeGua is 835 faster than that of 6tGua in water. We could be tempted to attribute this effect to the bigger SOC in Se than in S. However, we showed that SOC alone can speed up the decay by only a factor of 29. This perplexing observation prompts a deeper exploration into the underlying mechanisms governing this disparity.
The study unravels the intricate dynamics of the T1 decay process in these molecular systems. By computing the reorganization energy, activation energy, and spin-orbit coupling for T1/S0 Marcus rate calculation, we aimed to elucidate the molecular basis of the observed T1 lifetime difference. Integral to this investigation is the consideration of solvent effects.
Through a meticulous examination employing explicit microsolvation and implicit solvent models, we found that the hydrogen bond between the sulfur atom of 6tGua and water molecules plays a crucial role in modulating the triplet decay process. This interaction leads to an increased activation energy and acts as a brake, significantly prolonging the T1 lifetime of 6tGua compared to its selenium counterpart.
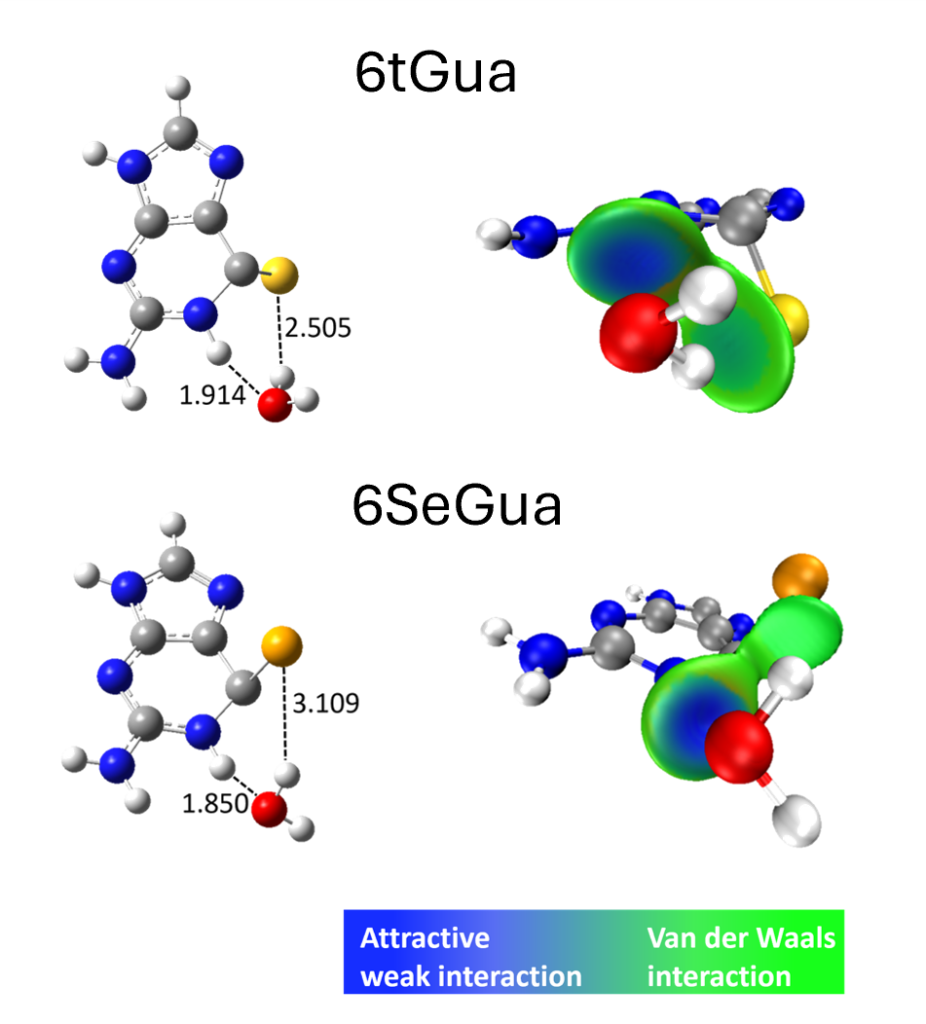
In contrast, the weaker interaction between 6SeGua and water molecules fails to exert a comparable effect. The activation energies are smaller, resulting in a much shorter T1 lifetime. This mechanistic insight not only elucidates the experimental observations but also underscores the critical role of solvents in shaping the dynamics of excited states.
Thus, we can factorize the different contributions to the T1 lifetime Se/S ratio (ΘSe/S= 835) into factors due to the reorganization energy (Θλ), activation energy (ΘΔE), and SOC (ΘSOC):
\Theta_{Se/S} = \Theta_\lambda\times\Theta_{\Delta E}\times \Theta_{SOC}We showed that reorganization energy is independent of solvent and plays a minor role (Θλ = 0.6). SOC is also solvent-independent and has a significant contribution (ΘSOC=29). Finally, activation energy is the most critical factor (ΘΔE=49) and is deeply dependent on the solvent.
The implications of these findings offer valuable insights for the design and optimization of photosensitizers tailored for specific applications, particularly in aqueous environments. By understanding the interplay between molecular structure, solvent interactions, and excited state dynamics, researchers can engineer photosensitizers with enhanced efficiency and efficacy.
In conclusion, the study highlights the intricate interplay between molecular structure, solvent dynamics, and excited state kinetics in dictating the efficiency of photosensitizers. By deciphering the mechanisms underlying the contrasting T1 lifetimes of 6tGua and 6SeGua, we hope to pave the way for future advancements in molecular design and application across diverse fields.
MB
Reference
[1] S. Liu, Y. Lee, L. Chen, J. Deng, T. Ma, M. Barbatti, S. Bai, Unexpected Longer T1 Lifetime of 6-Sulfur Guanine than 6-Selenium Guanine: Solvent Effect from Hydrogen Bond to Brake the Triplet Decay, Phys. Chem. Chem. Phys. (2024). DOI: 10.1039/d4cp00875h SMASH