Simulations show how to maximize reaction rates for singlet oxygen generation.
In brief:
- Reaction rates for 1O2 generation were computed with the DtC model.
- Rates were determined as a function of the geometry of the PS-O2 complex, spanning 15 different conformations.
- The rates mapped in this way change over five orders of magnitude between the largest and the smallest ones.
- 1O2 generation is maximized when O2 directly interacts with the PS region with highest spin density.
Singlet oxygen (1O2) is a central component for photodynamical therapy and diverse groups have been searching for the best photosensitizers (PS) to produce 1O2. In the most relevant channel in solution, 1O2 generation takes place through the reaction
1[3PS+3O2] → 1[1PS+1O2], (1)
in which a singlet complex (with each monomer in the triplet state) converts to another singlet state of the complex, but now with the spin of the monomers flipped to the singlet.
Shuming Bai and I have been developing a long-term project to explain the 1O2 formation photosensitized by thiothymines. Sooner this year, we proposed a computational model, name Dived-to-Conquer (DtC), to calculate kinetic rates for reaction (1). In our most recent work [1], we have employed the DtC model to investigate how the 1O2 generation rate depends on the spatial conformation of the PS-O2 complex.
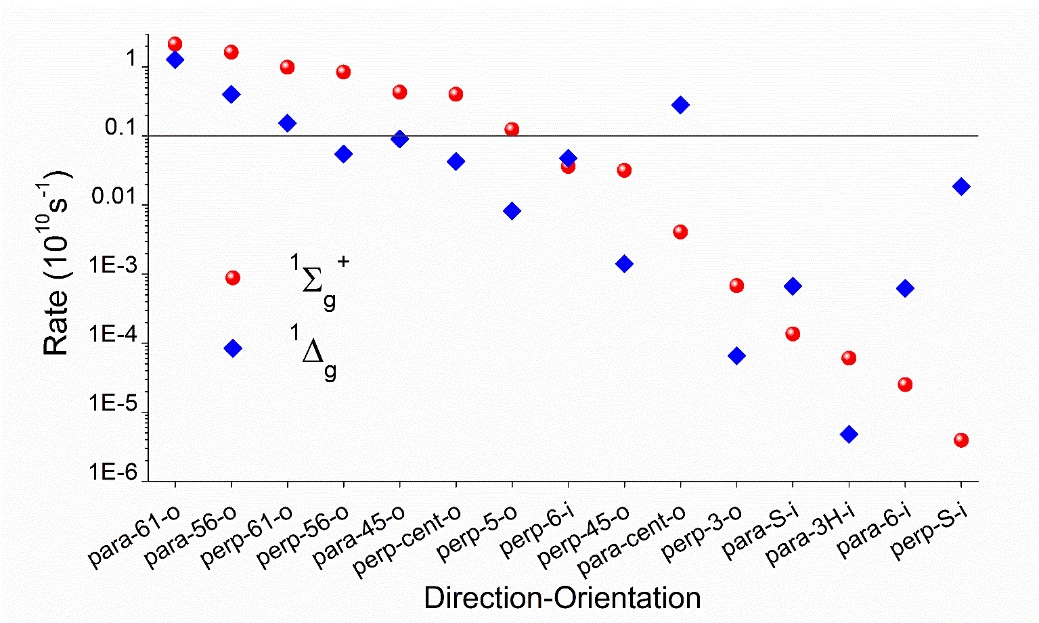
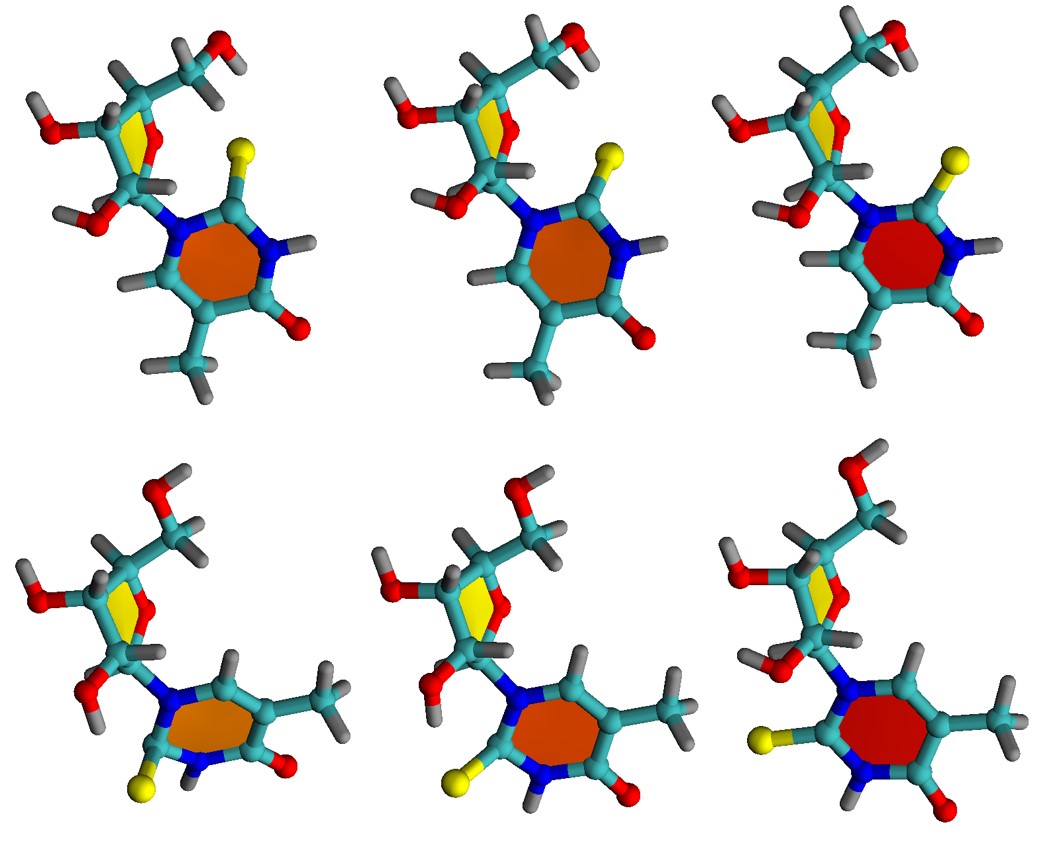
We mapped the rates for fifteen different directions and orientations for the incidence of O2 on PS. These directions span the whole conformational space around PS, allowing to know exactly which regions should maximize or minimize the singlet oxygen production. The rates, computed for 1O2 formation in the 1Σg+ and 1Δg states of oxygen, are shown in the figure below.
The rates strongly depend on the direction, spanning five orders of magnitude between the largest and the lowest. All productive directions feature out-of-plane (-o) incidence, having atoms C4/C5/N6 as targets, exactly where the highest spin density of triplet PS peaks. This finding implies that orbital overlap is a necessary condition for effective photosensitization and indicates the relative importance of different conformers. For 6-aza-2-thiothymine, for instance, singlet oxygen production is maximized for an O2 attack from above (or below) the aromatic ring. O2 attack on the ring plan (-i) renders low rates.
For the most productive directions, rates for forming 1Σg+ are larger than to form 1Δg by 10 to 60%. This means that the direct formation of the final singlet oxygen product (1Δg) should occur mainly via internal conversion from 1Σg+, but still with significant amounts of the direct formation.
The calculations also revealed that formation of 1O2 in the 1Σg+ and 1Δg states have different underlying mechanisms: 1Σg+ is formed with low activation energy and small diabatic couplings, while 1Δg is formed with high activation energies and large diabatic couplings.
These results were recently published in the J. Phys. Chem. Letters [1].
MB
Reference
[1] Shuming Bai and Mario Barbatti, Spatial Factors for Triplet Fusion Reaction of Singlet Oxygen Photosensitization, J. Phys. Chem. Letters doi:10.1021/acs.jpclett.7b02574 (2017).
1 Comment
Spin-Exchange Internal Conversion (SEIC) – Light and Molecules · February 21, 2019 at 6:11 PM
[…] A practical occurrence of reaction (2) is in the singlet-oxygen photosensitization. […]
Comments are closed.