Dynamics simulations of proton transfer
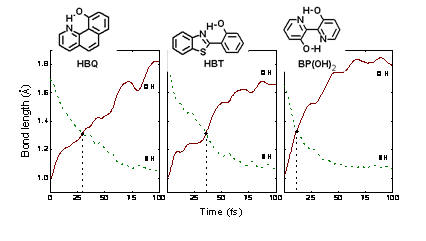
Many molecules undergo excited-state intramolecular proton transfer (ESIPT) as a result of an electronic excitation. This is the case, for instance, of 10-hydroxybenzo[h]quinoline (HBQ) (Ref), 2-(2′-hydroxyphenyl)benzothiazole (HBT) (Ref), and [2,2 ‘-Bipyridyl]-3,3 ‘-diol (BP(OH)2) (Ref).
In these three cases, the ESIPT dynamics is ultrafast and takes place in less than 100 fs.
We have performed excited-state dynamics simulations for the investigation of ESIPT processes. These simulations, carried out at TDDFT and CC2 levels, have helped to understand the structural deformations that trigger the proton transfer (Ref, see movie below).
Specifically, in the case of HBT in the gas phase, the dynamics simulations also predicted the occurrence of cis-trans isomerization after the ESIPT (see movie below), followed by internal conversion. This prediction has been confirmed by experiments also reported in Ref.
Dynamics simulations together with the experimental results from E. Riedle in the LMU (Munich) have allowed us to achieve a very complete view of the proton transfer cycle, including the regeneration of the initial molecule.
In the case of BP(OH)2 double proton transfer may occur. There is a discussion in the literature about whether the two transfers are simultaneous or sequential. The simulations clearly show that what has been experimentally determined as concerted transfer is, in fact, a combination of two sequential proton transfers separated by a small delay below the present experimental resolutions (Ref).
Now that ESIPT dynamics is getting clear, we can propose chemical designs to boost its yield and also use it in fluorescence markers.
We can even contribute to a long on-going debate in photochemistry, the nature of the double proton transfer in 7-azaindole dimer. (Spoiler alert: it’s not stepwise.)
Proton transfer within solvents
In collaboration with Nawee Kungwan, from Chiang Mai University, we have also been investigating excited state proton transfer within solvation.
These investigations are done using excited-state dynamics. In Ref, for instance, we run dynamics for 7-azaindole microsolvated with water at ADC(2) level.
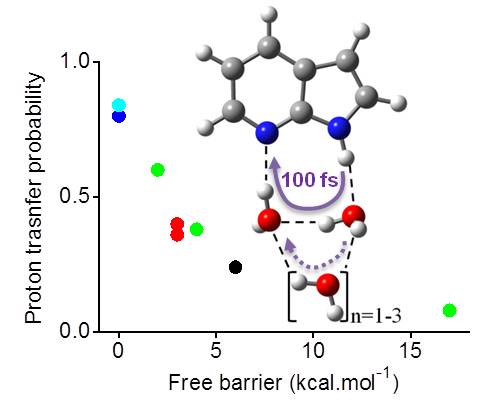
7-Azaindole (7AI) in a cluster with water (Ref).
We found out that the proton transfer happens mostly through the two nearest water molecules (it is, in fact, a double proton transfer). The remaining molecules in the second solvation shell do not take part directly. They have however an indirect effect of stabilizing the hydrogen bonds and reduce the proton-transfer energy barriers.
We have also investigated 1H-pyrrolo[3,2-h]quinoline in water and methanol (Ref) and 7-azaindolein water–methanol and methanol (Ref).