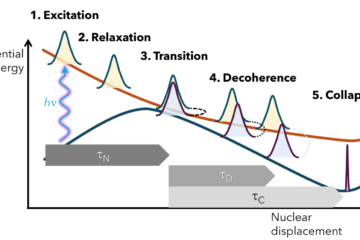
Work reviews what happens to DNA after UV excitations.
The interest in photoinduced effects in nucleic acids is deeply linked to the history of DNA itself. Less than one decade after Miescher isolated the “nuclein” in 1869, the bactericidal effect of UV radiation was discovered and became a fertile field of investigation.
When it was finally determined in 1928 that this lethal effect of UV radiation was due to its absorption by nucleic acids, it raised the first speculations that DNA could be related to growth and reproduction, a fact that would be finally confirmed only in 1944. In this meanwhile, DNA was thought to be a shell protecting proteins, thus preventing their dissociation by the action of UV light.
The determination of the molecular structure of DNA by Watson and Crick in 1953 paved the way for the discovery that photochemically-formed pyrimidine dimers cause biological damage. Since then, an immense effort has been dedicated to understand exactly how radiation and nucleic acids interact. The major reason for this interest rests, naturally, on health aspects: UV radiation is the main environmental agent triggering skin cancer.
Besides that, mutagenic effects of radiation have helped to shape life on Earth. The evolutionary selection of nucleic acids as hereditary agents was apparently induced by their physical-chemical response to the very high levels of UV radiation in the early-biotic world.
From these initial motivations, the research on photoinduced effects in nucleic acids points to a future where optical devices will take advantage of the unique charge-transport properties of these biological polymers.
Today, thanks to advanced spectroscopic and simulation techniques, we understand pretty well how nucleic acids react to UV radiation.
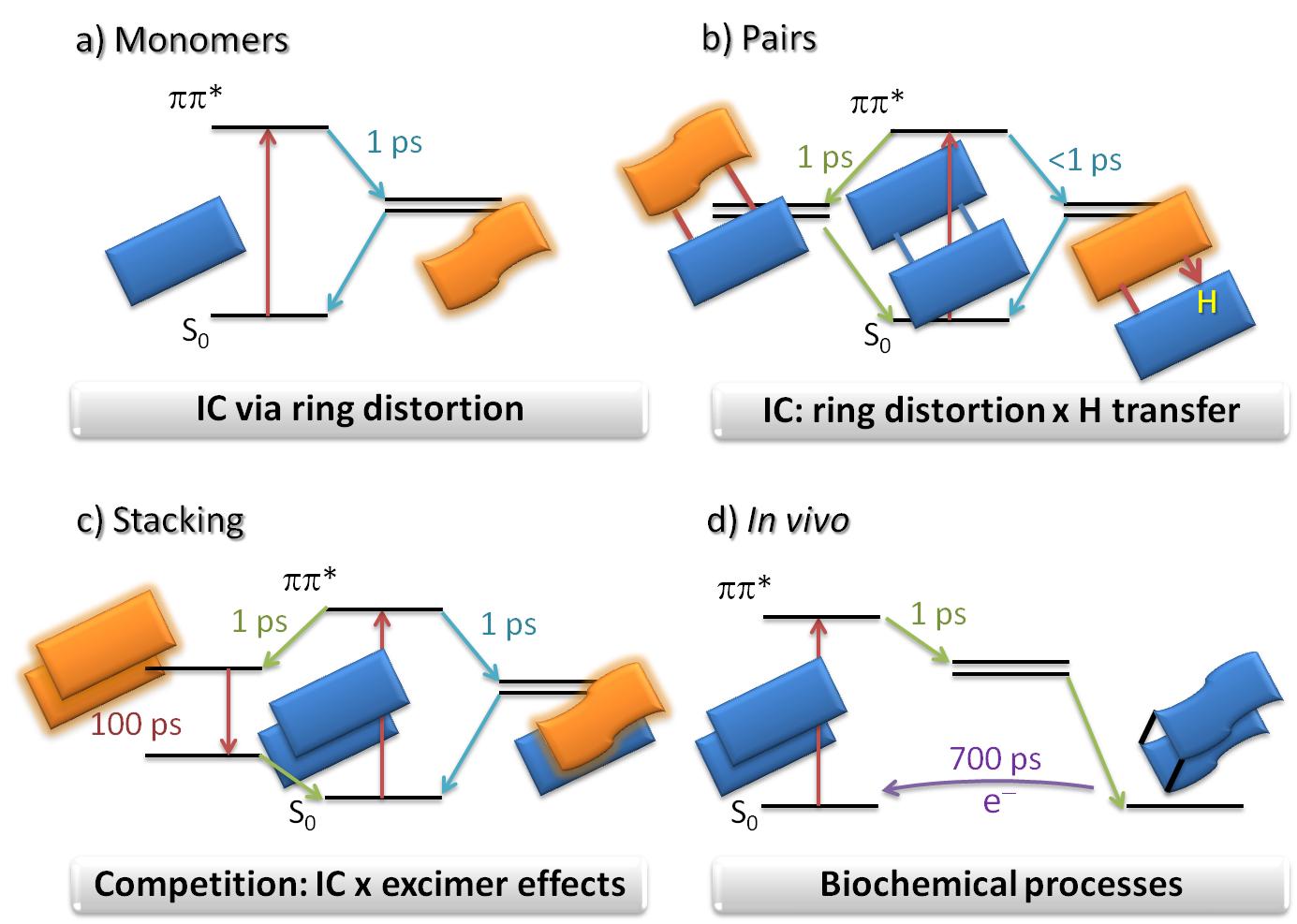 Isolated nucleobases, for instance, transform the radiation energy into heat by internal conversion (IC) caused by ring distortions (Figure a).
Isolated nucleobases, for instance, transform the radiation energy into heat by internal conversion (IC) caused by ring distortions (Figure a).
Base pairs, on their turn, also transform the radiation energy into heat, but not only by ring distortions. Hydrogen transfer between bases can do the job as well (Figure b).
In stacked fragments, internal conversion competes with excimer effects, which delocalize and trap the radiation energy in several neighbor nucleobases (Figure c).
In biological environments, besides all these processes, mutations and enzymatic reactions may also happen (Figure d).
If you want to know more about this topic, specially the physical-chemical aspects, I recommend to take a look at the review that Susanne Ullrich, Antonio Carlos Borin, and I have just published.
- M. Barbatti, A. C. Borin, S. Ullrich, Photoinduced processes in nucleic acids, in Photoinduced processes in nucleic acids, Vol. 1, edited by M. Barbatti, A. C. Borin, S. Ullrich, Top. Curr. Chem. doi:10.1007/128_2014_569 (Springer, 2014). doi:10.1007/128_2014_569