Phosphorescence of aromatic carbonyls takes much longer from ππ* than from nπ*. We explain why.
In brief:
- We explain theoretically why 3nπ* phosphorescence is faster than 3ππ* in aromatic carbonyls.
- The theoretical model was confirmed by numerical simulations for benzaldehyde derivatives using two different methods.
Since the 1960s, it known that the phosphorescence lifetime of aromatic carbonyl compounds is shorter for 3nπ* than for 3ππ* by a factor of five or bigger. This difference between phosphorescence lifetimes is often experimentally employed to assign the triplet state character. Nevertheless, the reason for existing such a significant difference in the first place is unknown.
The phosphorescence lifetime difference is often attributed to the El-Sayed rule. During the research for this article, I asked Twitter what the cause of this phenomenon would be. The El-Sayed rule was the most common answer, indicating this explanation’s popularity among the chemistry community. Nevertheless, despite exhaustive research, we could not locate any reference that showed how the El-Sayed rule would apply in this case.
We perused 65 years of literature unsuccessfully searching for a theoretical analysis of phosphorescence lifetime in aromatic carbonyls. Thus, we decided to fill this knowledge gap by surveying how molecular symmetry impacts phosphorescence from 3nπ* and 3ππ* in these molecules.
We show that the difference in the phosphorescence lifetimes is due to a selection rule related to (but more general than) the El-Sayed rule, controlling the coupling mechanism in each type of state. We explained this effect based on analyzing the first-order perturbative expansion of triplet-singlet transition dipole moments without considering vibronic couplings. We showed that the 3nπ*-S0 transition dipole moment depends on the permanent dipoles of the unperturbed S0 and T1, making it much bigger than the 3ππ*-S0 transition dipole moment, which depends on weaker transition dipole terms between unperturbed states.
In particular, the lifetime difference occurs if:
- the difference between the permanent dipole moments of S0 and T1 is significant and
- 3ππ* is totally symmetric.
We also show that it should strictly occur only for molecules attaining C2v and D2 symmetries, although it may occasionally be observed in other point groups. Finally, we discuss how vibronic effects impact the phosphorescence lifetimes.
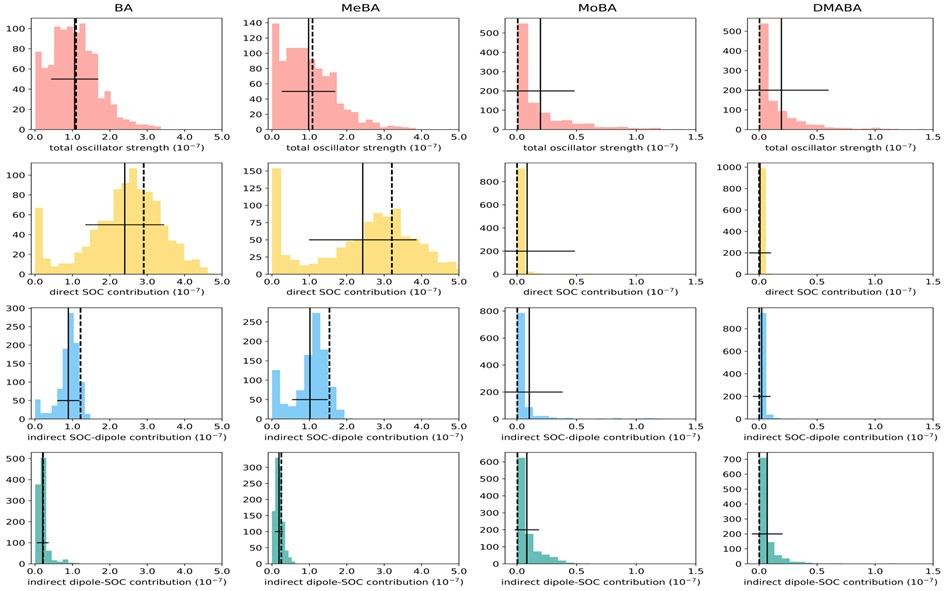
These predictions were verified with phosphorescence lifetime simulations of benzaldehyde and three benzaldehyde derivatives in the gas phase employing a vertical approximation and the nuclear ensemble approaches. Both predict 3nπ* emission within a few tens of milliseconds. While the vertical approach indicates a 3ππ* emission within a few seconds, vibronic corrections bring this value down to about 200 ms.
MB
Reference
[1] S. Mukherjee, M. Kar, M. Bhati, X. Gao, M. Barbatti, On the short and long phosphorescence lifetimes of aromatic carbonyls, Theor. Chem. Acc. (2023). DOI: 10.1007/s00214-023-03020-w