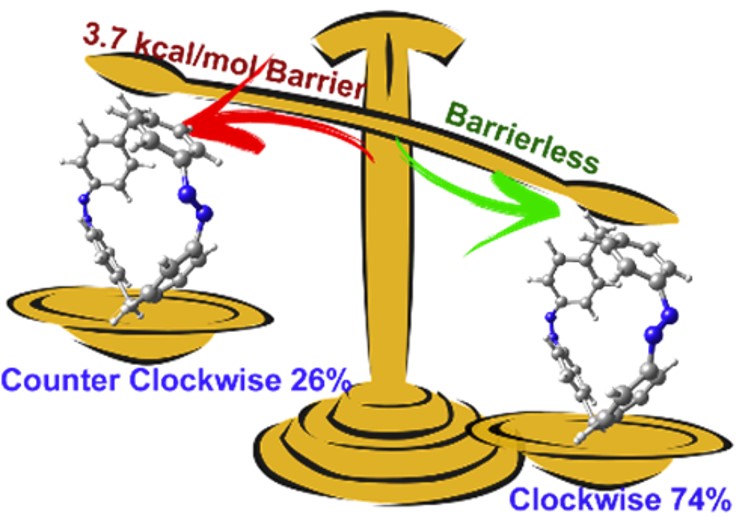
Cyclobiazobenzene twists clockwise in three out of four UV excitations.
In brief:
- Nonadiabatic dynamics simulation of cyclobiazobenzene (CBA) shows a strong preference for clockwise over counterclockwise photoisomerization.
- This stereoselectivity is caused by a 3.7 kcal/mol barrier along the counter-clock direction.
Full-dimensional nonadiabatic dynamics simulations based on OM2/MRCI semiempirical level have been used to explore the photoisomerization and subsequent excited-state decay of a macrocyclic cyclobiazobenzene (CBA) molecule [1].
Two S1/S0 conical intersections are responsible for the excited-state decay. They can be reached through either clockwise or counterclockwise rotational motions around the N=N bond.
In both pathways, the excited-state isomerization is ultrafast and finishes within about 69 fs; but dynamics simulations show that the clockwise isomerization channel is much more favorable (74%) than the counterclockwise one (26%).
The analysis of the potential energy along the pathways revealed that this stereoselectivity is caused by a 3.7 kcal/mol barrier along the counter-clockwise direction.
These results, obtained by Ganglong Cui’s group in Beijing Normal University, demonstrate that stereoselective pathways exist not only in the photoisomerization of isolated azobenzene-like systems but also in macrocyclic systems with multiple azobenzenes (ABs). Thus, we expect to provide useful insights for understanding and controlling the photodynamics of macrocyclic nanostructures with AB units as the core building units.
MB
Reference
[1] T.-S. Zhang, Z.-W. Li, Q. Fang, M. Barbatti, W.-H. Fang, and G. Cui, Stereoselective Excited-State Isomerization and Decay Paths in cis-Cyclobiazobenzene, J. Phys. Chem. A, doi: 10.1021/acs.jpca.9b04372 (2019).