UV-excited HCFC-133a eliminates Cl– through an ion-pair complex.
In brief:
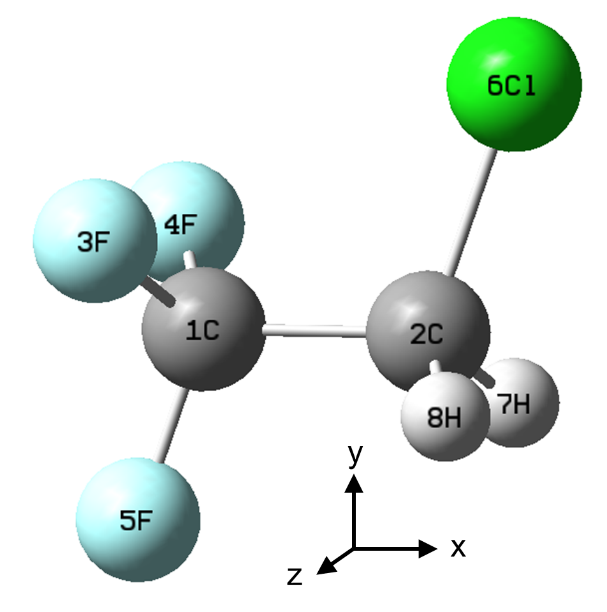
- MRCI shows that, after UV excitation, CF3CH2Cl (HCFC-133a) can form a highly polar ion-pair complex, CF3HCH+∙∙∙Cl–.
- This complex may be a source of Cl– fragments in this molecule.
- This hypothesis is confirmed by surface hopping dynamics at TDDFT level.
HCFC-133a (CF3CH2Cl) is a potent greenhouse and ozone-depleting substance of anthropogenic origin. Its removal from the atmosphere is partially done via UV irradiation, and a detailed description of its photochemistry should aid the modeling of its atmospheric effects.
In the last years, we have developed a research program studying the photochemistry of atmospheric halogenic compounds.
In the present work [1], we describe how HCFC-133a yields chlorine anions, which also has a great appeal to fundamental research due to the transient formation of a rare CH∙∙∙Cl bonded moiety. This research has been done employing state-of-the-art multireference methods and mixed quantum-classical nonadiabatic dynamics.
MRCI+Q calculations for the Cl dissociation shows a profile as in the figure below. There is a sequence of spots for nonadiabatic transitions that can populate the ion-pair state.
Surface hopping dynamics with decoherence-corrected fewest switches based on TDDFT shows that the ion-pair complex can indeed be formed in the sub-picosecond time scale.
The molecule reaches the ion-pair state with the complex in a symmetric structure, like that in figure (a) below, and later it tends to form the hydrogen-bond asymmetric structure (b).
This hydrogen-bonded structure, however, was not observed during the dynamics because the TDDFT jobs reach an S1/S0 crossing quickly after the ion-pair state is populated, and the simulations are terminated due to technical reasons.
The complex yield is strongly dependent on the excitation energy. Exciting the molecule at 10 eV produces only 4% of ion-pair complexes. Nevertheless, exciting the molecule at 11.2 eV yields 15% of ion-pair complexes.
These results are discussed in Ref. [1].
MB
Reference
[1] G. P. Rodrigues, T. M. L. de Lima, R. B. de Andrade, E. Ventura, S. A. do Monte, M. Barbatti, Photoinduced formation of H-bonded ion pair in HCFC-133a, J. Phys. Chem. A DOI: 10.1021/acs.jpca.8b12482 (2019).