Bezene excimers are formed within 1 ps and die after 0.3 ps.
In brief:
- Surface hopping dynamics of benzene dimer was simulated based on ADC(2) method.
- Starting from the parallel displaced geometry, an excimer is formed within 0.5-1 ps.
- Once formed, the excimer survives for 0.3 ps.
- The memory of which monomer was initially excited is lost within 1 ps.
Excimers are bound excited dimers without a bound ground state. Excimers and exciplexes (a heterodimer excimer) are important excited-state species involved in a number of photochemical and photophysical processes. They play crucial roles in optoelectronic properties of organic materials, underlying diverse phenomena such as fluorescence quenching, singlet-fission, and charge/energy diffusion.
The benzene dimer is a prototypical system for the study of excimers, for which there is an abundance of theoretical and experimental data. Its dynamics of formation and dissipation, however, is not well known.
(a) Parallel displaced, (b) T-shaped, and (c) parallel stacked (excimer) structures of benzene dimer.
In a project led by Thiago M. Cardozo, from the Federal University of Rio de Janeiro, we studied the nonadiabatic dynamics (S1 and S2 states) of isolated benzene dimers, with focus on the excimers [1]. The simulations were based on decoherence-corrected fewest-switches surface hopping, using ADC(2) electronic structure. The states accessed during the dynamics were characterized via transition density analysis.
The dynamics of excimer formation from parallel-displaced structures of an isolated benzene dimer can be summarized in the following way:
- After excitation into S1, the benzene rings move towards the excimer parallel-stacked structure (~0.5-1.0 ps), with ring distances reaching 3.0 Å and less.
- In the isolated dimer, the excess vibrational energy of ~1.0 eV doesn’t let the dimer to remain near the excimer potential well for too long, taking around 0.3 ps to leave that region.
- There are, however, recurrent visits to the excimer structure.
- The incoherent dynamics quickly leads to an even redistribution of the exciton localization between the two rings. The information about the initially excited monomer is lost after 1 ps.
(Left) Probability of excimer formation as a function of time. (Right) Distribution of the excimers’ lifetime.
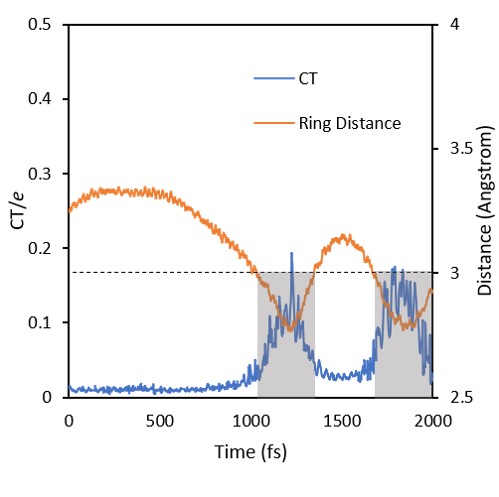
Out of the excimer region, the exciton tends to be localized in either one or other benzene, with often jumps between the two monomers. In the excimer region, delocalization and CT character increase.
Natural transition orbitals (NTO) for a regular exciton (parallel displaced, top) and for an excimer (parallel stacked, bottom).
In the isolated benzene dimer, with no possibilities to dissipate the excess excitation energy, one cannot form a stable excimer. Nevertheless, in extended systems or in the condensed phase, the hindered motion of groups, together with more pathways for vibrational energy redistribution may substantially elongate the excimer lifetime.
The movie below shows a typical trajectory. POS tells the exciton position (POS =1 or 2 = localized; POS = 1.5 = delocalized). CT tells the amount of charge transfer. Distance is the distance between the two benzene rings.
These results are published in Ref. [1].
MB
Reference
[1] T. M. Cardozo, A. P. Galliez, I. Borges Jr., F. Plasser, A. J. A. Aquino, M. Barbatti, and H. Lischka, Dynamics of Benzene Excimer Formation from the Parallel-Displaced Dimer, Phys. Chem. Chem. Phys., doi: 10.1039/c8cp06354k (2018).