Chemically different carbons cause dichroism in X-ray excitation of polyacenes.
In brief:
- The near-edge X-ray absorption of benzene, naphthalene, anthracene, tetracene, and pentacene was measured and simulated.
- They all have a sharp band at 284 eV.
- Increasing molecular size, a second band at 286 eV emerges and becomes the most intense.
- The two bands correspond to inner-shell excitations into chemically distinct carbon sites.
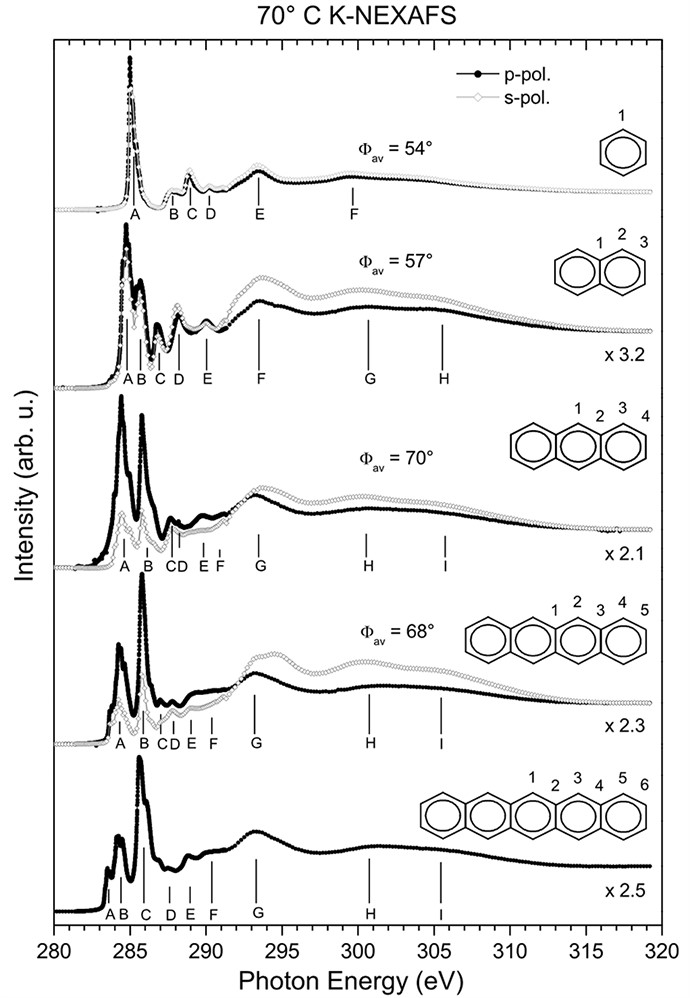
In an experimental and theoretical project, we have systematically studied the near-edge X-ray absorption fine structure (NEXAFS) spectra of films of a sequence of condensed acenes, including benzene, naphthalene, anthracene, tetracene, and pentacene.
The spectral complexity increases with the molecular size. All species show the first band at about 284 eV. (The energy position of the onset of the first resonance decreases from 284.86 eV for benzene to 283.26 eV for pentacene.) Nevertheless, when increasing the number of rings, a second band emerges at about 286 eV. The intensity of this second band increases systematically along the polyacene series, and it becomes the dominant one for anthracene and larger molecules.
To help in the assignment of the experimental spectra, the inner-shell spectrum of isolated polyacene molecules was simulated with the nuclear ensemble approach based on TDDFT electronic structure. Additionally, we analyzed the transition densities for these molecules.
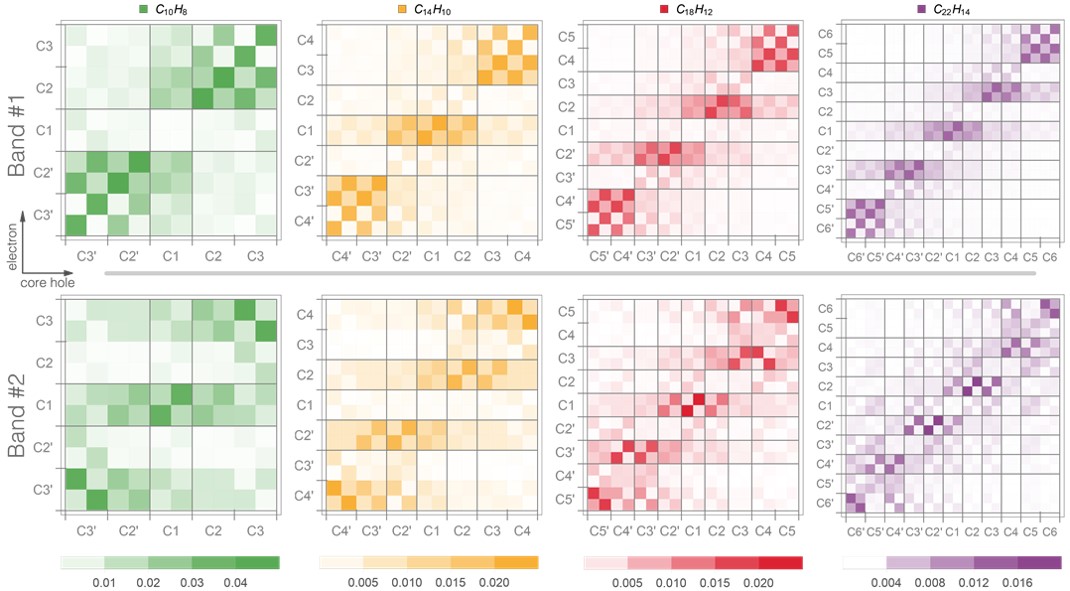
These simulations revealed that there are qualitative differences between the electronic transitions composing the first and second bands, in terms of electron/hole localization properties in chemically inequivalent carbons:
- In the first band, the values for the highest correlation are found for the mid-top and end-top carbon sites (taking anthracene, for example, these are the C1 and C3 atoms, respectively).
- In contrast, the transitions from the second band show the highest correlation for the mid-bottom and end-bottom sites (C2 and C4 atoms of anthracene, respectively).
Although the calculations could not perfectly reproduce the relative intensities of the bands, there is a reasonably good agreement between simulations and measurements in what concerns the relative position of the bands, which validate the potential of the current methodology as an effective way to explore inner shell excitations of large molecules.
These results were published in Ref. [1].
MB
Reference
[1] M. L. M. Rocco, M. Häming, C. E. V. de Moura, M. Barbatti, A. B. Rocha, A. Schoell, and E. Umbach, High-Resolution NEXAFS Study of Condensed Polyacenes, J. Phys. Chem. C, DOI: 10.1021/acs.jpcc.8b08945 (2018).