Multiscale photo-relaxation of thiouracil is resolved through theory and experiments.
In brief:
- The time-resolved photoelectron spectrum of 4-thiouracil is measured in the gas phase.
- With the help of theory, three time-constants are identified and assigned.
- ISC occurs between 1.8 and 3.3 ps depending on the pump wavelength.
- There are strong quantitative differences between time-constants of 4-thiouracil and 2-thiouracil.
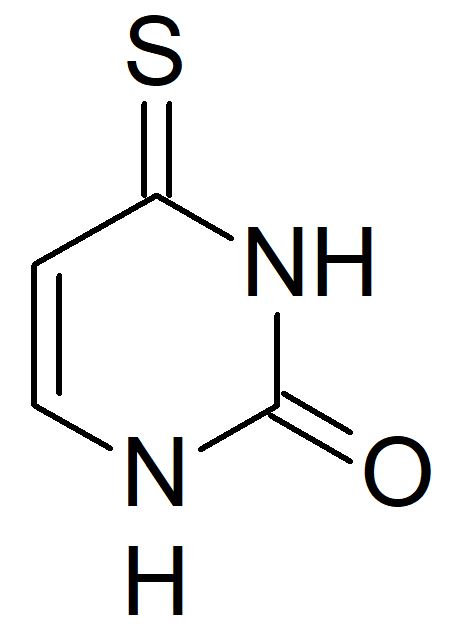
The UV photophysics of the natural and modified nucleobases can be surprisingly different. In response to UV radiation, the natural pyrimidine nucleobases undergo ultrafast internal conversion back to the ground state, whereas their thiobase analogs, in which an oxygen has been replaced by sulfur, display efficient intersystem crossing to the triplet manifold.
In collaboration with the experimental group of Susanne Ullrich, from the University of Georgia, we investigated the effect of the substituent position on 4-thiouracil (4-TU) in the gas phase using time-resolved photoelectron spectroscopy (TRPES) and computational modeling.
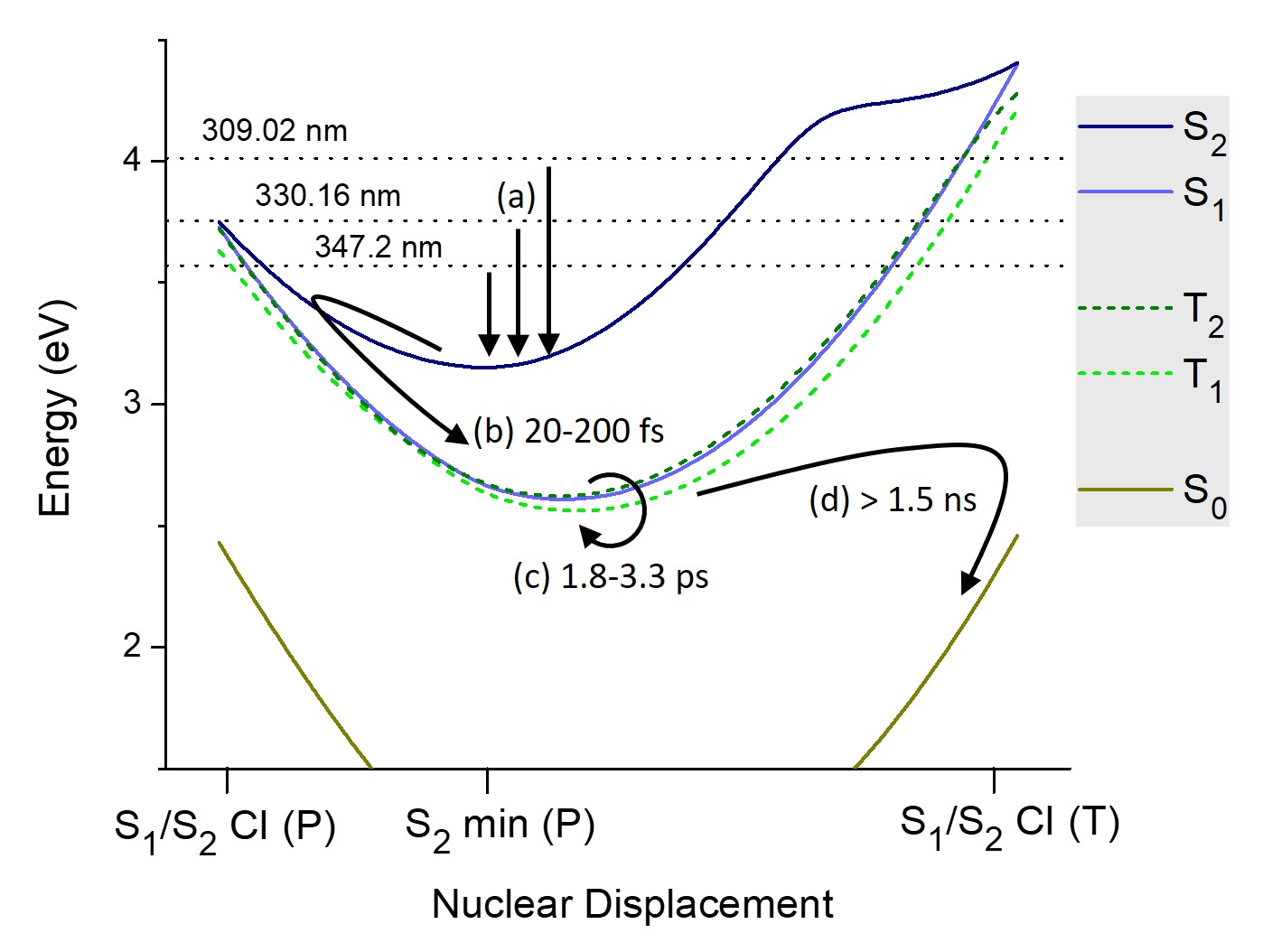
Measured with few different pump wavelengths, the TRPES spectrum of 4-TU shows three time-constants: the first one is ultrashort, in the range of 20 to 200 fs; the second time-constant is in the range from 1.8 to 3.3 ps; the final one is longer than 1.5 ns.
To assign these time-constants to specific steps in the excited-state relaxation, we analyzed the excited-state topography using ADC(2) computational method. We searched for ground- and excited-state minima, crossings between singlet states, and crossings between singlet and triplet states. Moreover, we determined the cation energies for each of these geometries.
All this information allowed to estimate the electron binding energy BE, which for a particular geometry R is given as
where the right side contains the energies of the pump laser, the cation Dn state (doublet), and the neutral Si state (singlet or triplet).
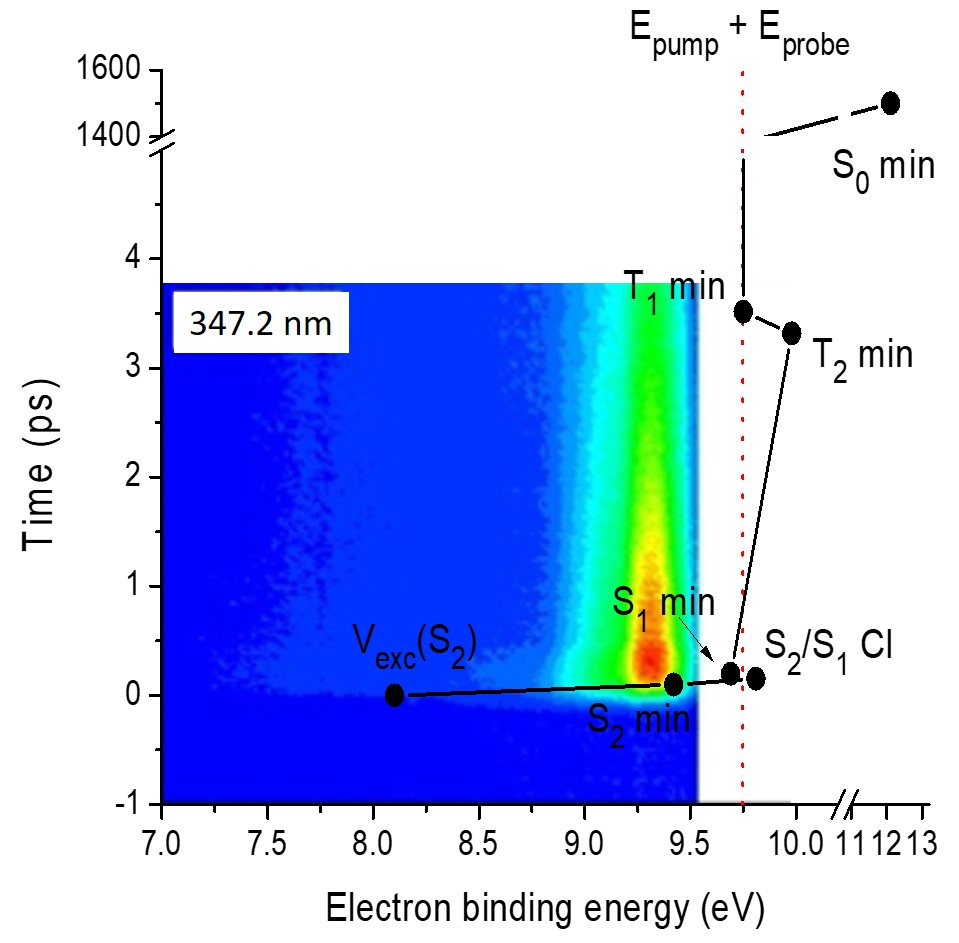
Electron binding energy calculated with ADC(2) (dots and lines) is superimposed to the experimental TRPES pumped at 347.2 nm. The time for each theoretical point is given by the experimental time-constants.
This simple estimate of BE allows to directly compare the experiments and theory, as done in the figure above.
The best match between experimental and theoretical BE is for:
- ultrafast relaxation to the S1 minimum corresponding to the first time-constant;
- intersystem crossing to T2 at a planar crossing within 1.8 to 3.3 ps (second time-constant);
- intersystem crossing to S0 after 1.5 ps.
This pathway is illustrated below.
Compared to 2-TU, whose TRPES has been reported before, the photophysics of 4-TU is qualitatively the same. Nevertheless, the time-constants are rather different: the ISC time-constant to populate the triplet states in 2-TU is much shorter than in 4-TU by about a factor 8. The ISC time-constant to populate the singlet ground state, which is longer than 1.5 ps in 4-TU, is only 109 ps in 2-TU. Thus, it’s clear that the immediate chemical environment of the sulfur atom plays a central role to ISC.
These results have been published in a special issue of Chemical Physics dedicated to Wolfgang Domcke [1].
MB
Reference
[1] A. Mohamadzade, S. Bai, M. Barbatti, and S. Ullrich, Intersystem crossing dynamics in thiouracils studied by time-resolved photoelectron spectroscopy: micro-environmental effects due to sulfur position; Chem. Phys. doi: 10.1016/j.chemphys.2018.08.011 (2018).