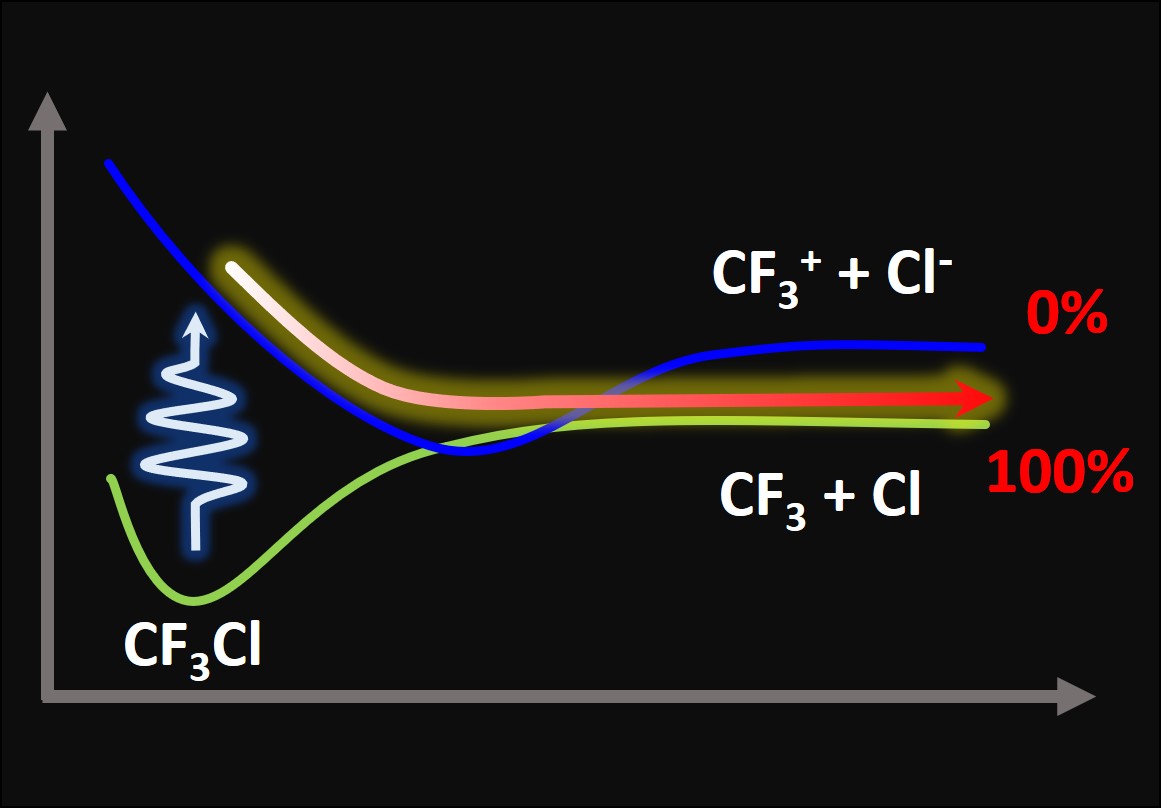
Anomalous charge quenching of CF3Cl is caused by internal conversion.
In brief:
- MR-CISD+Q is used to map the dissociation of CF3Cl into five channels.
- The only charged channel (CF3+ + Cl–) is disfavored by internal conversion to the ground state.
Although the CFC production has been discontinued by the Montreal Protocol, these substances are still a risk to the ozone layer. They not only have a long lifetime spanning a few hundred years, but there are indications that the protocol may have being breached in the last years.
In collaboration with my colleagues Silmar do Monte and Elizete Ventura, from the Federal University of Paraiba, we have been applying computational chemistry to characterize the photochemistry of these substances (as well as of HCFCs and Halons).
Recently, we worked on CFC-13 (CF3Cl) to map its ground and excited state dissociation channels. We used the multireference configuration interaction (MR-CISD+Q) method to describe the potential energy curves leading to CF3 and Cl fragments in the valence and Rydberg states.
In the lowest excitation region, these curves look like the figure below.
The mapping of the excited states of this molecule made clear that after UV excitation, it relaxes through a cascade of conical intersections, with possible dissociation through several channels.
Nevertheless, there is one odd feature about these states. The ion-pair state—in which the charged fragments CF3+ and Cl– are formed (the blue curve in the figure)—has a deep minimum caused by Coulomb interaction. This minimum is so stable that the potential energy curve of this state intersects the neutral ground state.
This piece of information seems just a side curiosity, but it, in fact, helps explain some puzzling information experimentalists have been observing for years in the fragmentation of halogenfluorocarbons like CF3Cl, CF3Br, and CF3I: after UV excitation, they do not produce charged fragments, as expected from a thermodynamic analysis.
Now we know the reason. Because the ion-pair state is super-stabilized and crosses the ground state, there is no way that CF3Cl could dissociate into the charged fragments. Everytime that the ion-pair state is formed, it preferentially converts to the ground state, dissociating into neutral fragments instead. The same is valid for the other halogenfluorocarbons.
These and many other findings about CFC-13 have been reported in Ref. [1].
MB
Reference
[1] V. C. de Medeiros, R. B. de Andrade, G. P. Rodrigues, G. F. Bauerfeldt, E. Ventura, M. Barbatti, and S. A. do Monte, Photochemistry of CF3Cl: Quenching of Charged Fragments is Caused by Nonadiabatic Effects, J. Chem. Theory Comp., DOI: 10.1021/acs.jctc.8b00457 (2018).