UV radiation triggers water evaporation within 30 fs.
In brief:
- Surface hopping photodynamics simulations at ADC(2) level is done for a cluster of thymine interacting with six water molecules.
- Compared to thymine in the gas phase, the destabilization of the nπ* state by hydrogen bonding enhances internal conversion from ππ* to nπ*.
- Internal conversion to the ground state is almost twice slower than in the gas phase.
When isolated thymine in the gas phase is excited by UV radiation, it quickly gets rid of the photoenergy by transforming it into heat.
However, what does happen if thymine is in water? Is the mechanism of energy dissipation still the same? Or does the water play some crucial role?
In a project led by Hans Lischka, we have addressed these questions using quantum-chemical simulations [1].
We have done excited-state dynamics simulations of thymine microsolvated by a cluster of six water molecules. 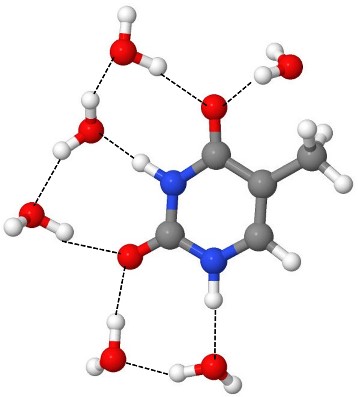
Dynamics was performed with decoherence-corrected surface hopping based on ADC(2) electronic structure.
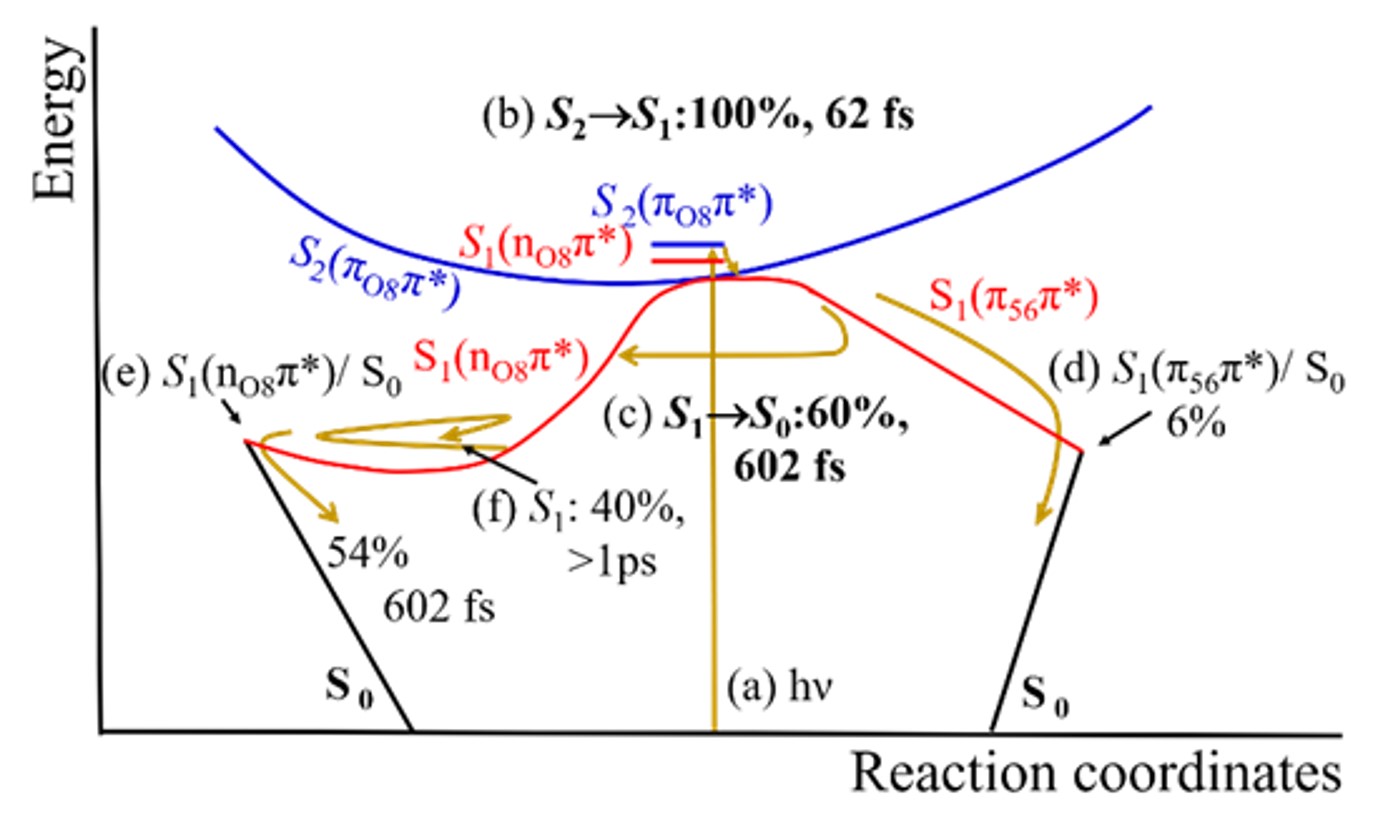
The results show that the qualitatively, there are not many differences between the energy dissipation for thymine in the gas phase and in the water cluster. After excitation in the ππ*, thymine still can quickly return to the ground state via two main conical intersections, one along the nπ* and another along the ππ* states.
Nevertheless, there are some significant differences between the gas phase and the cluster systems. In the water cluster, the nπ* state is destabilized due to the hydrogen bonds formed between water molecules and the nitrogen atoms of thymine.
As a consequence, the S2(ππ*) population is almost completely funneled to the S1(nπ*) state within only 62 fs. In the gas phase, this process takes 253 fs and involves only 84% of the population.
Moreover, because of this enhanced trapping in the S1(nπ*) state, internal conversion to the ground state is elongated from 391 fs in the gas phase to 602 fs in the cluster.
Time constants (fs) for the gas-phase thymine and for thymine/water cluster dynamics. Fi,i-1 is the fraction of the Si occupation decaying to Si-1 in the ultrafast regime.
| Thymine gas phase | Thy(H2O)6 cluster | |||
| Fi,i-1 | τi (fs) | Fi,i-1 | τi (fs) | |
| S2 → S1, i = 2 | 0.84 | 253 | 0.98 | 62 |
| S1 → S0, i = 1 | 0.70 | 391 | 0.61 | 602 |
Thus, it seems that water disturbs the system, but doe not really play an active role in the photochemistry, as we have seen in other cases.
Nevertheless, there is still an interesting additional feature in the cluster dynamics. The S1(nπ*) state is characterized by an electron transfer from the lone pair at the thymine’s carbonyl group (C=O) to the π system. This transfer disrupts the hydrogen bond network, and the two water molecules attached to the carbonyl fly away. This micro-evaporation starts just 30 fs after the photoexcitation.
The vibrational relaxation, the transfer of the photoenergy from the chromophore to the solvent, starts after 200 fs with a dissipation rate of 2 eV/ps.
These results have been published in a special issue of Chemical Physics dedicated to Wolfgang Domcke [1].
MB
Reference
[1] H. Lischka, M. Barbatti, F. Siddique, A. Das, and A. J. A. Aquino, The Effect of Hydrogen Bonding on the Nonadiabatic Dynamics of Thymine-water Cluster, Chem. Phys. DOI: 10.1016/j.chemphys.2018.07.050 (2018).