Photo-Fries rearrangement plays a central role in organic synthesis. But how does it really work?
In brief:
- Photo-Fries rearrangement (PFR) is controlled by three states: a ππ* absorbing the radiation; a pre-dissociative nπ* transferring energy to the dissociative region; and a πσ*, along which dissociation occurs.
- The lowest excited state has two minima, with ππ* and nπ* characters. The energetic balance between these minima controls the PFR yield.
Photo-Fries rearrangement (PFR)—a photochemical conversion of aryl esters to hydroxyphenones—is a key step in the synthesis of a large number of compounds. It also plays an important role in the design of functional polymers, and in the photodegradation of drugs and agrichemicals.
Given its chemical importance, it is surprising that the conceptual theoretical knowledge of this reaction is still incipient and even the full set of electronic states involved in the reaction has not been yet identified.
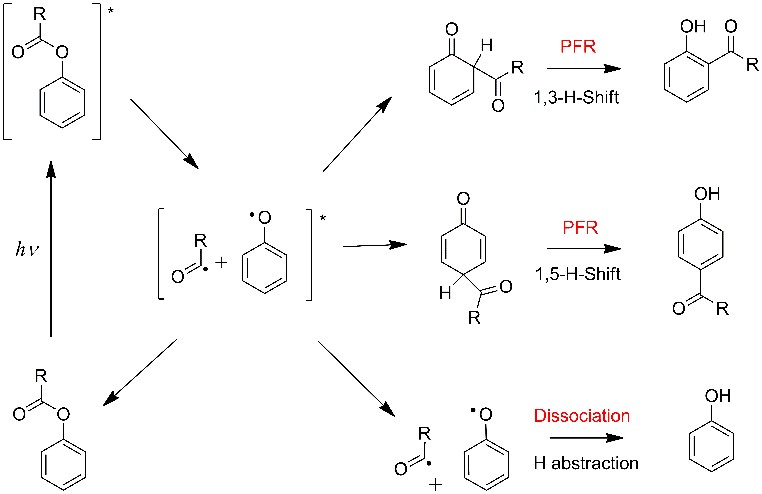
In a project led by Josene Toldo and in collaboration with Paulo F. B. Gonçalves from Federal University of Rio Grande do Sul (Brazil), we have built a mechanistic model to explain how the PFR takes place. Our aim has been to provide a comprehensive picture of the PFR, based on high-level multiconfigurational theoretical methods applied to a sequence of molecules of increasing complexity. This sequence spans from phenyl acetate, the minimum prototype to the PFR (R = methyl in the scheme above) all the way up to Porpoxur, an important insecticide (Baygon), whose UV photodegradation is a major environmental issue.
Our results revealed that the PFR involves three electronic excited states arranged along a specific topography that allows transferring the photoenergy from the aromatic to the carbonyl region.
The three states participating in the PFR are:
- an aromatic ππ*, which absorbs the radiation;
- a pre-dissociative nπ*, which transfers the energy to the dissociative region;
- and a πσ*, in which dissociation occurs.
The transfer from ππ* to nπ* involves pyramidalization of the carbonyl carbon, while the transfer from nπ* to πσ* takes place through CO stretching. Different products are available after a conical intersection with the ground state. Among them, there is a recombined radical intermediate, which can yield ortho-PFR products after an intramolecular 1,3-H tunneling.
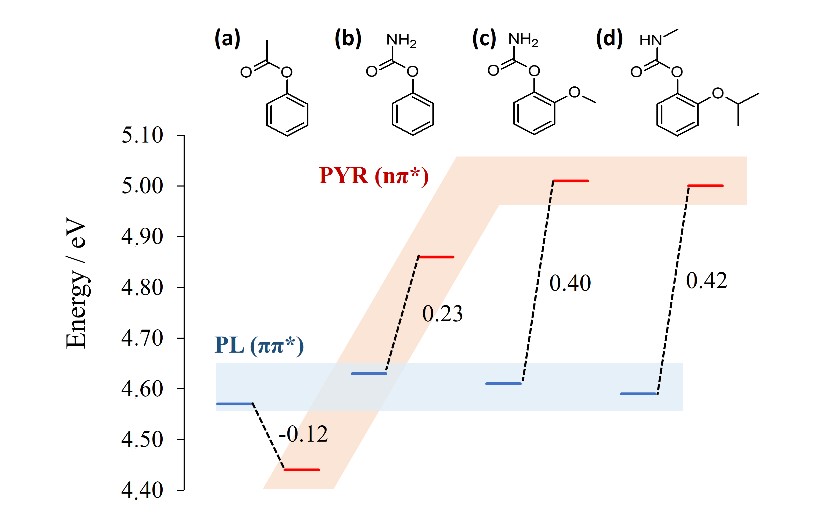
The three-state model for the PFR is able to reconcile theory with a series of observations from time-resolved spectroscopy. It also delivers a rational way to optimize the PFR yields, since diverse substituents or solvents can change the energetic order of the ππ* and nπ* states, preventing or enhancing the reaction.
This energetic order is the crucial factor for the PRF. If the ππ* is more stable than the nπ* state, it is not possible to transfer energy to the dissociation. In this case, the PFR is inhibited. On the other hand, if ππ* is less stable, energy can be transferred, increasing the PFR yield.
These results have been recently published in Ref. [1].
MB
Reference
[1] Josene M. Toldo, Mario Barbatti, and Paulo F. B. Gonçalves, A three-state model for the Photo-Fries rearrangement, Phys. Chem. Chem. Phys. doi:10.1039/C7CP03777E (2017).