Sulfur bridge’s oxidation state dramatically changes the photoluminescence of bichromophores. That’s why.
In brief:
- Recent experiments showed that the luminescent intensity of a dimer composed of two naphthalenes connected by a sulfur bridge strongly depends on the oxidation state of the bridge (S, SO, or SO2).
- This dependence is caused by internal conversion processes, whose rates are controlled by the bridge’s oxidation state.
- For S and SO, paths to conical intersections are available, reducing the luminescence. For SO2, the conical intersection disappears, bumping up the fluorescence quantum yield.
Bichromophores—covalent dimers of organic chromophores—is a class of compounds that has been explored for organic electronics. These molecules are expected play multiple functions, as to help prevent charge recombination at donor/acceptor interfaces; or to optimize photoluminescence in OLEDs.
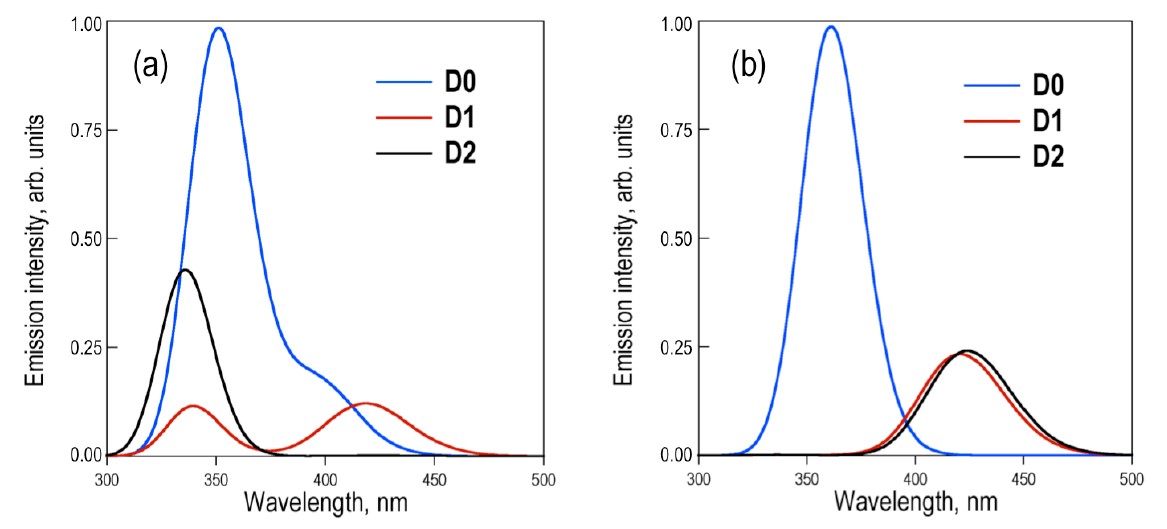
The applicability of bichromophores is related to the nature of the bridged monomers and how they interact. Recently, it was experimentally shown that the covalent link between conjugated chromophores by a sulfur bridge has a large impact on the fluorescence efficiency of the parent chromophores [1]. For example, in the case of two naphthalenes bridged by a SOn group (n = 0, 1, or 2; see figure below), the fluorescence quantum yield grows slightly from 0.012 for n = 0 to 0.035 for n = 1; and then it jumps to 0.27 for n =2.
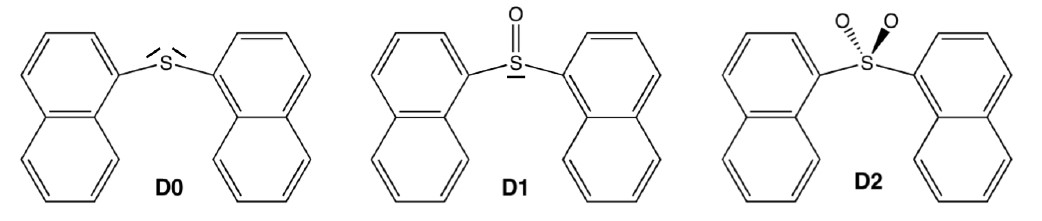 Although this finding revealed a clear strategy in the search for strong molecular emitters, it also asked for rationalization of its underlying photophysics.
Although this finding revealed a clear strategy in the search for strong molecular emitters, it also asked for rationalization of its underlying photophysics.
In this project, executed by Clàudia Climent from the University of Barcelona and led by David Casanova from the University of the Basque Country, we have applied computational-chemistry modeling to explain why the oxidation state of the sulfur bridge changes the photoluminescence of a bichromophore [2]. To do so, we worked on the same three SOn sulfur-bridged naphthalene dimers.
Our first hypothesis was that the oxidation state of the bridge affected the transition dipole moments and, therefore, the emission properties. To verify this hypothesis, we studied the conformers’ distribution for the dimer.
Depending on the excitation conditions, two limit cases may occur, either (a) initial excitation doesn’t change the conformer population and the emitting states are entirely controlled by the ground state equilibrium, or (b) the final emitting states are controlled by the relative stability of excited-sate minima. Either way, the photoluminescence of the three species are strongly affected, as shown in the next graph. This variation is caused by the different amounts of charge that flows to the sulfur bridge in the excited state, changing the transition dipole moment.
But the impact of the oxidation state on the transition dipole moments doesn’t agree with the experiments: taken this effect alone, the D0 molecule should have the highest emission among the three species, exactly the opposite from what the measurements show.
Therefore, we investigated an alternative hypothesis; we supposed that internal conversion to the ground state was the responsible for the effect.
In fact, the analysis of the reaction pathways for each species showed that while conical intersections between the nσ* and the ground state are available for compounds D0 and D1, they aren’t for D2. And that’s due to the simple reason that there are no n orbitals left in the D2 bridge.
This finding perfectly matches the experimental results: due to the conical intersections, D0 and D1 should have low and similar fluorescence quantum yields, while D2, lacking a conical intersection, should have large fluorescence quantum yield.
Thus, the photoluminescence dependence on the sulfur bridge’s oxidation state in naphthalene dimers arises from nonadiabatic processes that deplete the excited-state population in D0 and D1, but not in D2. The oxidation state of the sulfur-bridging atom directly influences the photoluminescence of the dimer by enhancing or depriving radiative and non-radiative relaxation pathways. In particular, oxidation controls the amount of electronic transfer between naphthalene moieties and the participation of the sulfur bridge in the low-lying electronic transitions.
(By the way, if internal conversion hypothesis didn’t explain the experiments, we would still have a third one to test: intersystem crossing.)
These results are discussed in a recent publication in Chemical Science [2].
MB
References
[1] P. R. Christensen, J. K. Nagle, A. Bhatti, and M. O. Wolf, Enhanced Photoluminescence of Sulfur-Bridged Organic Chromophores, J. Am. Chem. Soc. 2013, 135, 8109 (2013).
[2] C. Climent, M. Barbatti, M. O. Wolf, C. J. Bardeen, and D. Casanova, Photophysics of Naphthalene Dimers Controlled by the Sulfur Bridge Oxidation, Chem. Sci doi:10.1039/C7SC01285C (2017).