Double-well shaped triplet state in thionucleobases can be used to tune singlet oxygen yields.
In brief:
- The lowest triplet state of thionucleobases has two ππ* minima; an intersection to the singlet ground state is accessible from only one of them.
- The relative energy of these minima controls the lifetime of the triplet state, by modulating the intersystem crossing probability.
- This basic photophysics is shared by thiothymines, thiocytosines, and thioguanines.
UV irradiation of thionucleobases has been in the spotlight of medical research due to its use for cancer phototherapy as well as due to its carcinogenic effect on patients taking these substances as an immunosuppressant.
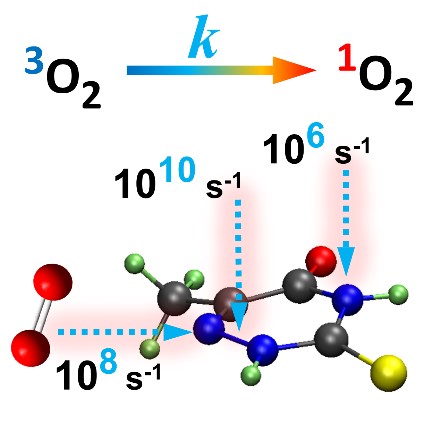
In both cases, the key chemical reaction is the singlet oxygen production induced by UV-excited thionucleobases. Ideally, when designing new drugs for either application, we should be able to tune the singlet oxygen yield between zero when dealing with an immunosuppressant and the unity when aiming at phototherapy.
Shuming Bai and I are proposing that the answer to this tuning problem may be in the control of the intrinsic triplet decay of thionucleobases [1].
Based on CASPT2//CASPT2 modeling of prototypical thionucleobases (2-thiothymine, 6-azo-2thiothime, 2-thiocytosine, and 6-thioguanine), we found out that the lowest triplet state of these molecules decays through a two-step mechanism; and that the singlet oxygen yield may be tuned up or down depending on which of these steps determines the decay rate.
Here it is why:
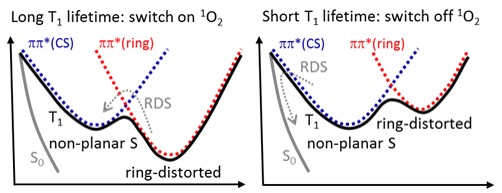
All these molecules have a T1 state characterized by a double well topography, caused by two strongly coupled 3ππ* states (see Figure below). One of the 3ππ* states has a minimum for a ring-distorted geometry, while the other has a minimum for a non-planar S geometry. Moreover, an intersection between T1 and S0 lies near the non-planar S geometry.
The relative energy of the two 3ππ* minima depends on the particular molecule, and two possible limit cases are shown in the graphs above.
When the ring-distorted minimum is more stable than the non-planar S minimum (left graph), intersystem crossing is disfavored and the T1 state has a long lifetime. When, however, the non-planar S is the most stable (right graph), then, intersystem crossing is favored and the T1 state has a relatively short lifetime.
This intrinsic decay dynamics of the T1 state impacts the singlet oxygen production: long T1 lifetime means high singlet oxygen yield (because it gives enough time for an encounter with an O2 molecule and for the spin change); short T1 lifetime, on its turn, means low singlet oxygen yield for exactly the opposite reason.
We have verified these findings by re-analyzing a series of previous experimental results, and showing that the two-step mechanism is perfectly consistent with chemical kinetics and transient spectroscopy of thionucleobases.
In practical terms, if we want substances with low singlet oxygen yield (to be used as an immunosuppressant, for example), we should look for derivatives with stable non-planar S minimum. If, however, we want substances with high singlet oxygen yield (to be used for phototherapy), we should look for derivatives with a stable ring-distorted minimum.
These results have been published in a recent paper in PCCP [1].
MB
Reference
[1] S. Bai and M. Barbatti, On the Decay of the Triplet State of Thionucleobases, Phys. Chem. Chem. Phys. doi:10.1039/C7CP02050C (2017).
- More about thionucleobases in Explaining the photophysics of thiothymines.
2 Comments
A kinetic model for singlet oxygen photogeneration | Light and Molecules · October 12, 2017 at 8:16 AM
[…] On the decay of the triplet state of thionucleobases […]
Singlet oxygen: rates strongly depend on geometry | Light and Molecules · October 26, 2017 at 7:27 AM
[…] On the decay of the triplet state of thionucleobases […]
Comments are closed.