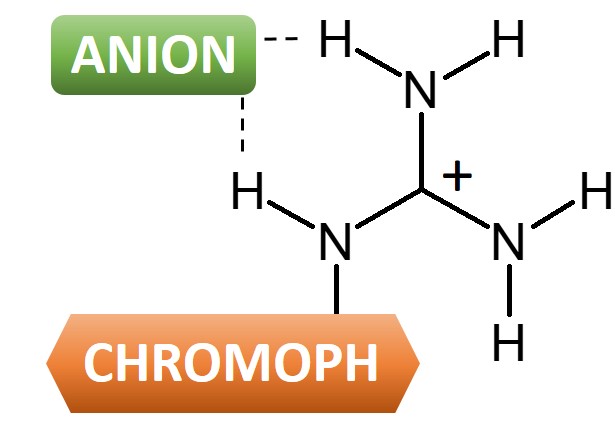
Chromophore-guanidine compounds can be used as selective anion sensors.
In a research project led by Ivana Antol, from the Ruđer Bošković Institute in Zagreb, we have explored anion binding properties of aromatic guanidines, with focus on their UV/vis spectroscopy [1].
This joint experimental-computational research aims at the design of efficient selective anion sensors based on chromophore-guanidine derivatives. The idea is to add these molecular sensors to a solution that may contain unknown anionic species. As soon as an anion binds to guanidine (forming a guanidinium…anion salt), the absorption spectrum of the chromophore attached to the guanidine group shifts. If this shift is selective enough, we may use this property to tell not only about anions present in the solution, but also about their nature.
To test the spectral response of these systems, we have computed UV/Vis spectra of several chromophore-guanidine derivatives and of its complexes with anions, in the gas phase and in acetonitrile, using TDDFT. The results compose a comprehensive spectroscopic benchmark, including six aromatic chromophores (phenyl, naphthyl, anthracenyl, quinolinyl, anthraquinonyl, and coumarinyl) and five anions (CF3SO3ˉ, NO3ˉ, HCOOˉ, CH3COOˉ, and Fˉ).
We observed that protonation of guanidine subunit shifts the lowest energy absorption bands toward higher energies. The shift is then reduced upon complexation with anions.
In phenylguanidine salts, in particular, the spectral shifts are correlated to anion basicity and H-bonding strength. This is a proof of principle that chromophore-guanidine compounds can be used as selective anion sensors. This selectivity, however, tends to diminish upon increase of chromophore size (naphthyl, anthracenyl).
This work was recently published at the Journal of Physical Chemistry A [1]. For more information on the excited states of guanidine and guanidinium alone, check this post.
Reference
[1] I. Antol, Z. Glasovac, D. Margetić, R. Crespo-Otero, and M. Barbatti, Insights on the Auxochromic Properties of Guanidinium Group, J. Phys. Chem. A, doi:10.1021/acs.jpca.6b05180 (2016).