Why does replacing different oxygens of thymine with sulfur cause distinct absorption and intersystem crossing?
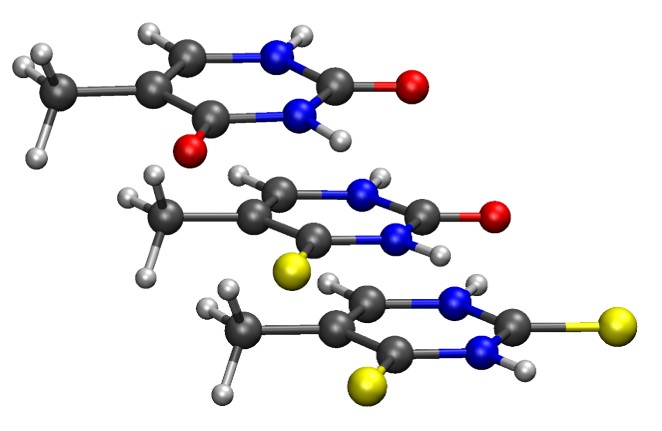
Recent experiments replacing oxygen atoms by sulfur in thymine revealed that absorption and intersystem crossings (ISC) properties of these derivatives are strongly dependent on the position and number of substitutions [1]. This dependence affects the performance of thiothymines for photodynamical therapy and may help to explain their carcinogenic effects when used as immunosuppressants.
Shuming Bai and I have been studying this relation between structure and photophysics. Using multireference quantum chemical methods (CASPT2 and DFT/MRCI), we computed absorption spectra and spin-orbit coupling (SOC) matrix elements for thymine (Thy), 2-thiothymine (2tThy), 4-thiothymine (4tThy), and 2,4-dithiothymine (2,4dtThy).
These simulations allowed us to address the following points unveiled by the experiments:
- Single or double substitutions lead to strong absorption red shift in relation to Thy.
- In the spectral region above 250 nm, 2,4dtThd has a multi-structured spectrum, while the other three species show a single band.
- The red shift is much larger in 4tThy than in 2tThy.
- The absorptivity of 2tThd is almost twice as large as that of 2tThy.
- The ISC lifetime of 4tThy is significantly shorter than that of 2tThy.
- The ISC lifetime of Thy is surprisingly short, only 0.76 ps.
I am not going into details here, but our spectrum simulations showed that a simple 4-electrons/4-orbital minimum model can explain points 1, 2, and 3. (In fact, we showed that point 1 can be explained even by Hückel model.) The analysis of these four molecular orbitals clarifies how replacing oxygen with sulfur in different positions has different effects on electron density delocalization and orbital energies, with direct consequences to the spectra.
Point 4 is still an unsolved mystery left: in spite of our several different attempts of modelling the larger absorptivity of 2tThd, we could not find any satisfactory explanation.
We addressed points 5 and 6 by computation of SOC elements for singlet-triplet transitions at CASPT2 level.
It is particularly interesting that these simulations revealed that the experimental ISC lifetime of Thy in water is underestimated by a factor 20. This happens due to an overlap of singlet/triplet absorption signals in the transient absorption spectrum, not accounted for in the experimental analysis.
Therefore, I am afraid we have spoiled the wonder in point 6: Thy ISC lifetime should be much longer than the experimentally proposed value.
These results were recently published in the Journal of Chemical Physics A [2].
References
[1] M. Pollum, S. Jockusch, C. E. Crespo-Hernández, 2,4-Dithiothymine as a Potent Uva Chemotherapeutic Agent, J. Am. Chem. Soc. 136, 17930 (2014).
[2] S. Bai and M. Barbatti, Why Replacing Different Oxygens of Thymine with Sulfur Causes Distinct Absorption and Intersystem Crossing, J. Phys. Chem. A (2016), doi: 10.1021/acs.jpca.6b05110.