Excited states of bromites are true challenges for theoretical chemistry. It’s time to face them.
When methyl hypobromite (CH3OBr) is excited, it quickly dissociates producing a Br radical. Simple to say, difficult to show. The quantum chemical description of this dissociation process is extremely challenging.
In a project led by Ljiljana Stojanović and in collaboration with our colleagues from the King Abdulaziz University, we have been working on the description of the excited states of this molecule [1]. After building a comprehensive benchmark including MR-CISD, CCSD(T), CCSD, CC2, ADC(2), and TDDFT, we figured out that none of these methods could fully describe CH3OBr.
The single-reference methods (CCs, ADC, and TDDFT) failed to provide reasonable dissociation limits. MR-CISD, on its turn, provided nice dissociation curves, but delivered a poor description of the bright σσ* state.
Moreover, the presence of Br, a relatively heavy atom, leads to non-negligible spin-orbit coupling effects. For instance, the intensity of the first absorption band if spin-orbit couplings are not considered is wrong by a factor two compared to the experiments.
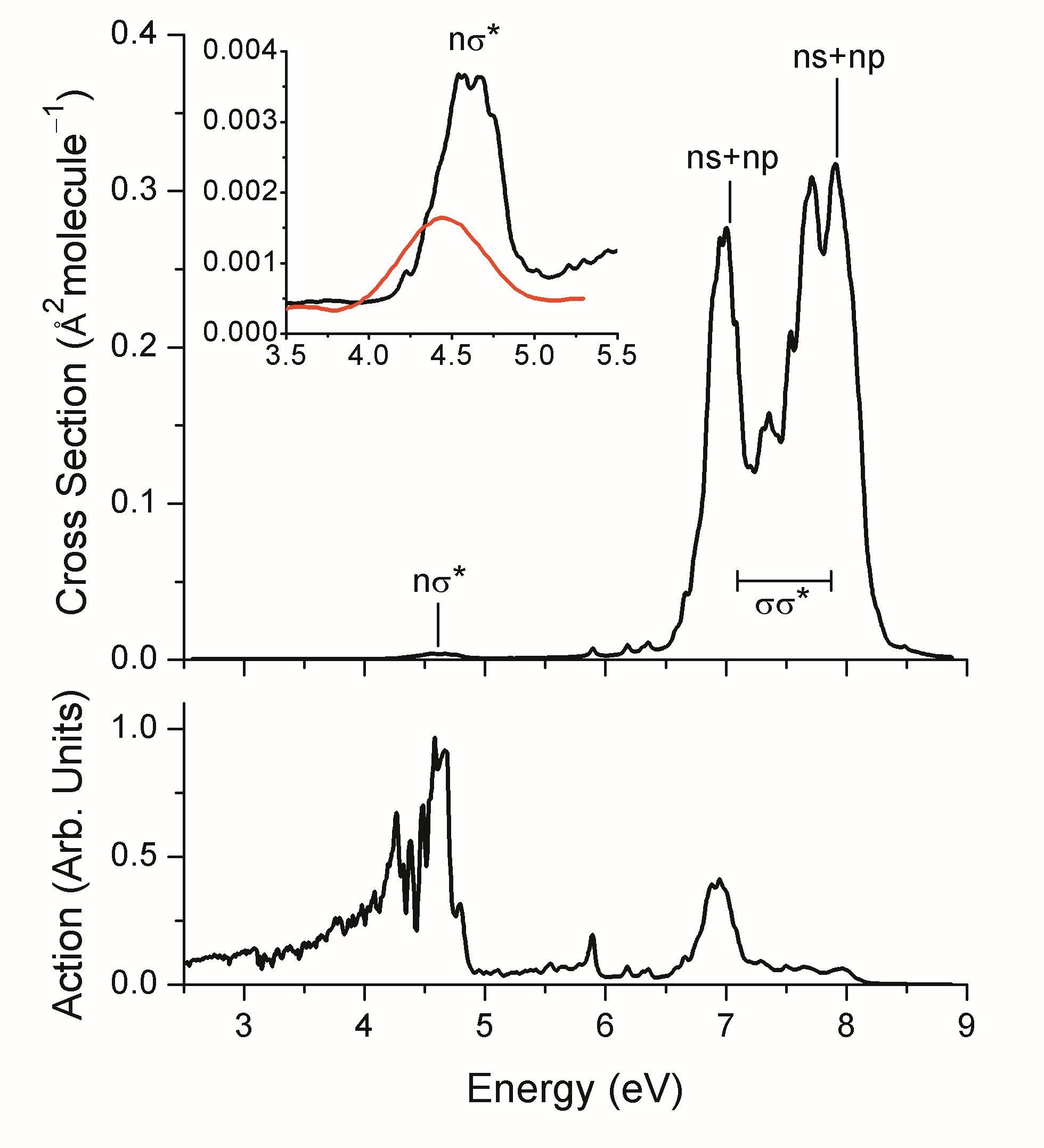
In spite of all these challenges, we have been able to provide a full description of the absorption spectrum and dissociation of methyl hypobromite. As shown in the figure above, the molecule strongly absorbs above 6.6 eV due to σσ* and Rydberg states. It also has a very weak band due to nσ* transitions at 4.5 eV.
When we multiply this spectrum by the solar irradiance at the Earth’s surface, we get the action spectrum, which shows that this weak nσ* band is the main responsible for the photochemistry of CH3OBr in the environment.
Reference
[1] L. Stojanović, G. Pereira Rodrigues, S. G. Aziz, R. H. Hilal, and M. Barbatti, Photochemistry of methyl hypobromite (CH3OBr): excited states and photoabsorption spectrum, RSC Adv., doi:10.1039/C5RA18578E (2015).
2 Comments
Nonadiabatic Dynamics of HCFC-132b | Light and Molecules · November 29, 2015 at 8:41 AM
[…] Photochemistry of methyl hypobromite […]
The Halons-9 Molecular Set | Light and Molecules · November 11, 2016 at 7:22 PM
[…] question hit us recently when we started looking into the photochemistry of HCFC and brominated compounds. In spite of their importance for green-house effect and ozone depletion, we quickly […]
Comments are closed.