Excited state proton transfer in 7-azaindole in water takes place through the first solvation shell.
Nawee Kungwan (Chiang Mai University) and I have been collaborating in the last years to understand how excited-state proton transfer takes place when a molecule is place in solvent.
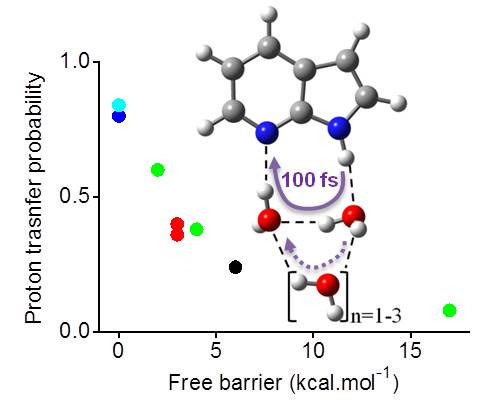
These investigations are done using excited-state dynamics. In our most recent publication [1], we run dynamics for 7-azaindole microsolvated with water at ADC(2) level.
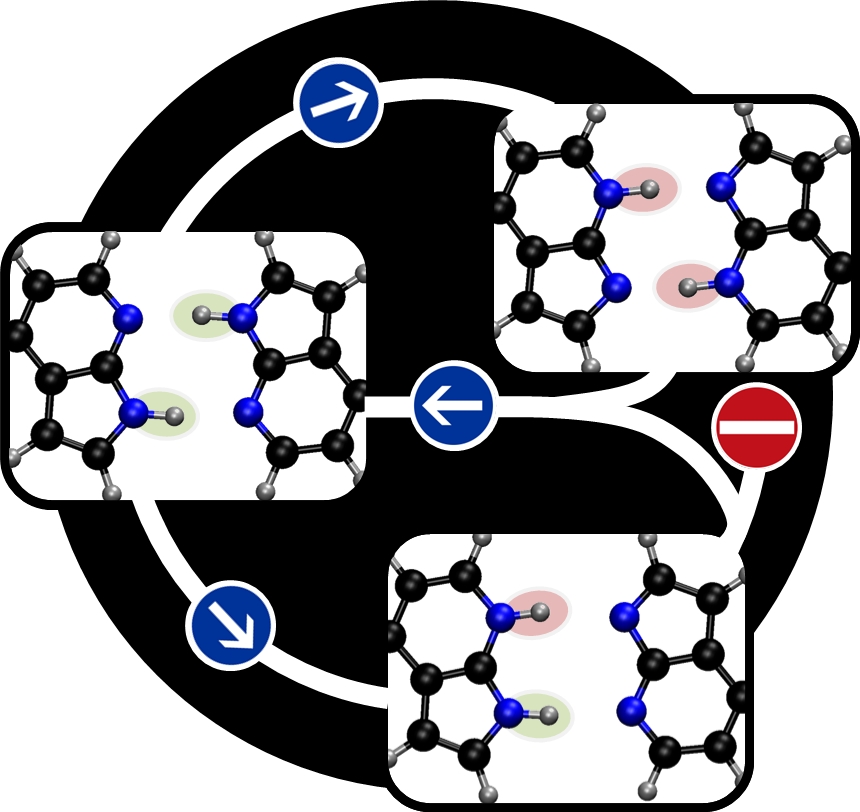
We found out that the proton transfer happens mostly through the two nearest water molecules (it is in fact a double proton transfer). The remaining molecules in the second solvation shell do not take part directly. They have however an indirect effect of stabilizing the hydrogen bonds and reduce the proton-transfer energy barriers.
Reference
N. Kungwan, K. Kerdpol, R. Daengngern, S. Hannongbua, M. Barbatti, Effects of the second hydration shell on excited-state multiple proton transfer: Dynamics simulations of 7-azaindole:(H2O)1-5 clusters in the gas phase; Theor. Chem. Acc. 133, 1480 (2014).
doi:10.1007/s00214-014-1480-y